Tree Diversity and Carbon Stocks of Hmuifang Forest, Mizoram
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

World Bank Document
h -- Public Disclosure Authorized gn,un,r- s' t .S *K t ' t~~~~~~~~~~-- i ll E il P \~~~t 4 1- ' Public Disclosure Authorized (na'g HS) zY Wm"y''''S.'f' ;', ', ''' '',''-' '~'0', t'' .SC:''''''''E 3'; , 'r' 6 ~ U Public Disclosure Authorized it ~ ~ ~ ~ ~ Public Disclosure Authorized OA 86b3 ' :~~~~~~~~~~~ TABLE OF CONTENTS 1. INTRODUCTORY BACKGROUND ................................................... 1-1 1.1. PROJECT BACKGROUND ................................................... 1-1 1.2. PROPOSED WORKS FOR BP1 -THE AIZAWL BYPASS . ..................................1-1 1.3. IMPACTS ENVISAGED AND THE CORRIDOR OF IMPACT . ..............................1-4 1.4. SCOPE OF ENVIRONMENTAL IMPACT ASSESSMENT ................................... 1-6 1.5. THE STUDY METHODOLOGY ................................................................... 1-6 1.6. STRUCTURE OF THE REPORT ................................................................... 1-7 2.' POLICY, LEGAL AND ADMINISTRATIVE FRAMEWORK . ..............................2-1 2.1. IMPLEMENTATION AND REGUALTORY AGENCIES .......................................................... 2-1 2.2. LEGAL FRAMEWORK AND THE ENVIRONMENTAL CLEARANCE STIPULATIONS ............ 2-1 2.3. GOI/GOM CLEARANCE REQUIREMENTS ..................................................................... 2-2 2.4. WORLD BANK REQUIREMENTS ..................................................................... 2-2 3. THE EXISTING ENVIRONMENT ..................................................................... 3-1 3.1. METEOROLOGICAL CONDITIONS -

Seventh Legislative Assembly of Mizoram (Twelfth Session)
504 SEVENTH LEGISLATIVE ASSEMBLY OF MIZORAM (TWELFTH SESSION) LIST OF BUSINESS FOR NINTH SITTING ON FRIDAY, THE 24th MARCH, 2017 (Time 10:30 A.M. to 1:00 P.M. and 2:00 P.M. to 4:00 P.M.) QUESTIONS 1. Questions entered in separate list to be asked and oral answers given. PRIVATE MEMBERS’ BUSINESS 2. * Resolutions NGURTHANZUALA Secretary __________________________________________________________________ * Please see Bulletin Part-II No. 170, dated the 20th March, 2017 for relative precedence of Private Members’ Resolutions to be moved. S P E A K E R : Mi fel ka chuan finna thu a sawi thin a, a lei chuan rorel felna thu a sawi bawk thin. Sam 37 :30 505 Tunah zawhna kan la ang a, zawhna no.104-na neitu Pu Nihar Kanti Chakma, MLA zahawm tak i sawm ang. Pu NIHAR KANTI CHAKMA : Ka zawhna Starred Question no 104-na Local Administration Department Minister zahawm tak chhan atan – Veng hrang hranga public toilet kan neih te tifaitu tur bik dah theih dan a awm em? S P E A K E R : Department changtu Minister zahawm tak Pu P.C Lalthanliana chhang turin i lo sawm ang. Pu P.C LALTHANLIANA, MINISTER : Pu Speaker, member zahawm tak Pu Nihar Kanti Chakma zawhna Starred Question 104-na chhanna chu public toilet enkawl lai Department in kan nei hranpa lo. Enkawltu dah theih a ni lo tih a ni e. S P E A K E R : Zawhbelhna, Pu Nihar Kanti Chakma. Pu NIHAR KANTI CHAKMA : Pu Speaker, ka lawm e. Hemi hi ka ngai pawimawh em em a, kan hriat fo angin India ram pumpuiah Swachh Bharat uar takin a kal chho mek a, Mizoram-ah pawh tha takin kan kal chhoh mek laiin, hmun hrang hrangah vantlang zunin te a bikin public toilet, department tin ten eng eng emaw hi an sa nual a, a rimchhe em em mai a, kei chuan hmantlakah pawh ka ngai lo a ni. -

Report of the Comptroller and Auditor General of India
Report of the Comptroller and Auditor General of India for the year ended 31 March 2010 (Report No. 2) GOVERNMENT OF MIZORAM TABLE OF CONTENTS Paragraph Page Preface v Overview vii CHAPTER-I PERFORMANCE REVIEWS RURAL DEVELOPMENT DEPARTMENT Border Area Development Programme 1.1 1 FOOD, CIVIL SUPPLIES AND CONSUMER AFFAIRS DEPARTMENT Public Distribution System 1.2 29 CHAPTER-II AUDIT OF TRANSACTIONS Wasteful/Temporary Misappropriation/Avoidable/Diversion/Idle Expenditure PUBLIC WORKS DEPARTMENT Wasteful expenditure 2.1 59 INDUSTRIES DEPARTMENT Temporary misappropriation/non-accountal of Government money 2.2 61 PUBLIC WORKS DEPARTMENT Loss due to undue financial aid to contractors 2.3 62 DISTRICT COUNCILS AFFAIRS DEPARTMENT Undue financial benefit to contractor 2.4 63 SOCIAL WELFARE DEPARTMENT Grant of old age pension to ineligible beneficiaries 2.5 65 LAND REVENUE AND SETTLEMENT DEPARTMENT Avoidable expenditure 2.6 67 RURAL DEVELOPMENT DEPARTMENT Unauthorised diversion of Central assistance 2.7 68 DISTRICT COUNCILS AFFAIRS DEPARTMENT Unauthorised diversion of recurring grants 2.8 69 LAND REVENUE AND SETTLEMENT DEPARTMENT Drawal of funds before actual execution of project 2.9 70 ANIMAL HUSBANDRY AND VETERINARY DEPARTMENT Idle expenditure 2.10 72 Regularity issues and other points General Follow up of Audit Reports 2.11 74 Response to audit observations and compliance thereof by the 2.12 75 Executive Audit Report (Civil) (Report No. 2) for the year ended 31 March 2010 Paragraph Page CHAPTER-III INTEGRATED AUDIT Integrated Audit of Higher and -

Cfreptiles & Amphibians
HTTPS://JOURNALS.KU.EDU/REPTILESANDAMPHIBIANSTABLE OF CONTENTS IRCF REPTILES & AMPHIBIANSREPTILES • VOL & AMPHIBIANS15, NO 4 • DEC 2008 • 28(2):189 280–284 • AUG 2021 IRCF REPTILES & AMPHIBIANS CONSERVATION AND NATURAL HISTORY TABLE OF CONTENTS NewFEATURE LocalityARTICLES Records of the Zunheboto . Chasing Bullsnakes (Pituophis catenifer sayi) in Wisconsin: HornedOn the Road to Understanding Frog, the Ecology Xenophrys and Conservation of the Midwest’s Giant zunhebotoensis Serpent ...................... Joshua M. Kapfer 190 . The Shared History of Treeboas (Corallus grenadensis) and Humans on Grenada: MathewA Hypothetical and Excursion Sen ............................................................................................................................ 2007 (Anura: Megophryidae:Robert W. Henderson 198 RESEARCH ARTICLES . The Texas HornedMegophryinae), Lizard in Central and Western Texas ....................... Emily from Henry, Jason Brewer, IndiaKrista Mougey, and Gad Perry 204 . The Knight Anole (Anolis equestris) in Florida .............................................Brian J. Camposano, Kenneth L. Krysko, Kevin M. Enge, Ellen M. Donlan, and Michael Granatosky 212 Samuel Lalronunga1,2, Malsawmdawngliana2,3, Lalkhawngaiha Sailo2, Vanlalvuana4, and Esther Lalhmingliani1,2 CONSERVATION ALERT 1Systematics and Toxicology Laboratory, Department of Zoology, Mizoram University, Aizawl 796004, Mizoram, India ([email protected]) . World’s Mammals in Crisis 2...............................................................................................................................Wildlife -

Ado)3Fl (Tisl 'S~Aol3 Aqiuewunooa Ieu!6Hjoh
'Ado)3fl (tisl 's~aoL3 AqIuewunooa ieu!6hJOh- Aq!PGS!lAGJt Public Disclosure Authorized Public Disclosure Authorized . ........... Z,, .... V V '4% 4±. 4"~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~..... Public Disclosure Authorized V..~~~~~~~~~~...... "C..~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~..... Public Disclosure Authorized [ wflOA ...... 96V3 ;uow;~~~~~~~~~~~~~~~~~~~~~~~~~~~~~edao ~~~~~~~~~~~~~~~~jqn~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~ ~ ~ ~ ~ ~ ~ ~ ~ ~ ~~ ~...... WUJOZIWjo UQWUSWAO9~~~~~~~~~~~~~~~~~~~~~~~~. .. .. I PREFACE The Mizoram State Roads Project includesaugmentation of the capacityand structural upgradationof selectedroad network in the state. A total of 185.71kmroads will be improved/upgraded,and major maintenanceworks will be carriedout on 518.615kmroads, in 2 Phases.The project was preparedby the ProjectCo-ordinating Consultants (PCC)', on behalf of the PWD, Mizoram.As part of the project preparation,environmental/social assessmentswere carriedout, as requiredby the WorldBank and the Governmentof India. In accordanceto the requirementsof the WorldBank, the environmental/socialassessments (and the outputs) had been subjectedto an IndependentReview. The independentreview 2 evaluatedthe EA processesand outputsin the projectto verify that (a) the EA had been carriedout withoutany biasor influencefrom the projectproponent and/or the PCC,(b) the EA/SAhad beenable to influenceplanning and design of the project;and (c) the outputs, especiallythe mitigation/managementmeasures identified in the EA/SA processesare | adequatefor the -

Mizoram Public Works Department RP77 Volume 2
Government of Mizoram Public Works Department RP77 Volume 2 ,- :-, - -- f:. Public Disclosure Authorized MizoramState Roads Project -47;~~~~~~7 Public Disclosure Authorized Resestlementand Indigenous Peoples DevelopmentPlan (Phasea4) 34 u Ann.ur.s Public Disclosure Authorized October2001 Public Disclosure Authorized (Original Documentby [CT CES,LBII) TABLE OF CONTENTS ANNEXURE-2.1 SCHEDULE FOR CENSUS SURVEY ANNEXURE-2.2 SCHEDULE FOR SOCIO-ECONOMIC SURVEY ANNEXURE-2.3 WORLD BANK FUNDED SOCIAL & ENVIRONMENT IMPACT ASSESSMENT ANNEXURE-3.1 MIZORAM STATE HIGHWAY RESETTLEMENTAND REHABILITATION POLICY ANNEXURE-3.2 APPROVAL OF MIZORAM STATE HIGHWAY RESETTLEMENT AND REHABILITATION POLICY ANNEXURE-7 DETAILS OF VARIOUS CATEGORIES OF IMPACTED PROPERTIES ALONG PRIORITY ROAD PlA ANNEXURE-8.1 PUBLIC INFORMATION & CONSULTATIONS AIZWAL-LUNGLEI (VIA HMUIFANG) - 100 KM ANNEXURE-8.2 PUBLIC INFORMATION & CONSULTATIONS AND GENERAL EXIBHITS- PHOTOGRAPHS ANNEXURE-8.3 COVERAGE OF PUBLIC CONSULTATIONS IN THE LOCAL MEDIA ANNEXURE-9.1 LAND SETTLEMENT AND THE ACTS OF MIZORAM REGULATIONS AND RULES GOVERNING REVENUE ADMINISTRATION ANNEXURE-9.2 DETAILED STATEMENT OF LANDS OF ORGANISATION/ASSOCIATIONS ETC., REQUIRED FOR IMPROVEMENT & UPGRADATION OF AIZAWL-THENZAWL- LUNGLEI ROAD (VIA HMUIFANG) ANNEXURE-9.3 DETAILED STATEMENT OF LANDS AVAILABLE WITHIN RESPECTIVE VILLAGE COUNCIL JURISDICTIONS & FREE LANDS REQUIRED FOR IMPROVEMENT & UPGRADATION OF AIZAWL-THENZAWL-LUNGLEIROAD ANNEXURE-9.4 DETAILED STATEMENT OF GOVERNMENT DEPARTMENT LANDS REQUIRED FOR IMPROVEMENT & UPGRADATION -

The List of Protected Monuments by Department of Art & Culture
ART & CULTURE DEPARTMENT GOVERNMENT OF MIZORAM Archaeology Section Under-mentioned is the list of protected monuments by Department of Art & Culture under Section 3 of Mizoram Ancient Monuments and Archaeological Sites and Remains Act, 2001. 1. Ancient arts Chhawrtui, Champhai District 2. BakPuk Lungrang ‘S’, Lunglei District 3. BengkhuaiaThlan Sailam, Aizawl District 4. Bung Bungalow Tuirial, Aizawl District 5. Captain Brown-a Thlan Changsil Fort, Aizawl District 6. ChhuraLungkawh Sihfa, Aizawl District 7. Chief Saihleia Residence Samlukhai, Aizawl District 8. DullaiSial Hliappui, Champhai District 9. DurtlangLal In Durtlang, Aizawl District 10. FiaraTui Farkawn, Champhai District 11. HualtuNgamtawnaUi No neihna Hualtu, Serchhip District 12. Kawlkulh Bungalow Kawlkulh, ChamphaiDistirct 13. KhuangzangPuk&Mitdelhnuhthlakna Phuaibuang, Aizawl District 14. Kolasib Bungalow Kolasib, Kolasib District 15. Kumpinu Lung Saitual, Aizawl District 16. LalLalvungaThlan Sazep, Champhai District 17. LalLungdawh Phulpui, Aizawl District 18. Lalbengkhuaia& Sap Sa-UiTanna Serchhip, Serchhip District 19. Lalhrima Bung lehTuikhur Thenzawl, Serchhip District 20. LallulaHmun Samthang, Champhai District 21. LalnuRothluaiiThlan Leite, Lunglei District 22. Lalruanga Lung Khawbung, Champhai District 23. LalruangaLungkaih Suangpuilawn, Aizawl District 24. LamsialPuk Farkawn, Champhai District 25. LianchhiariLunglentlang Dungtlang, Champhai District 26. Lung hmainei Tawitlang, Aizawl District 27. Lungau Ruallung, Aizawl District 28. Lungkeiphawtial Farkawn, Champhai District 29. Lungkhawdur Vanchengpui, Champhai Dist. 30. Lungkhawsa Vaphai, Champhai District 31. LungphunLian Lungphunlian,Champhai Dist. 32. Mel venghmasaber Hualngohmun, Aizawl District 33. MeltlangPuk Thingsat, Aizawl District 34. Mission School Serkawn, LungleiDIstrict 35. Mura Puk Sialhawk, Aizawl District 36. Mura Puk Zote, Champhai District 37. NeihlaiateNupathlan Hmuifang, Aizawl District 38. Ngente Nu LunglenTlang Khawrihnim, Mamit District 39. North Vanlaiphai Dispensary N. Vanlaiphai, Champhai Dist. -

The Mizoram Gazette EXTRA ORDINARY Published by Authority REGN
, -, ' • 'I' The Mizoram Gazette EXTRA ORDINARY Published by Authority REGN. No. NE. 313 (Mz) ----_._- .._---_._----,-,--------- NOT I FICATION No. LAD/VC-II/97, the 25th September, 2002. Mizoram Governor chuan Lushai Hills District (Village Councils) Act. 1953 (as amended in 1986) section 2 of clause (20) na hmangin Larnchhip Village Boundary chu Annexure a sawi flab ang hian a siam a ni. ( Lalthuamliana, Under Secretary to the Govt. of Mizeram. LAMCHHIP VILLAGE BOUNDARY Khawthlang lam Hmuifang tlang Dolala lungphun kawn starting point a hmangin ramri lui hmarchhak hawi zawnga luang zawh thlain, Khuailui zawh thla zclin Tuiritai lui hmar hawi zawngin a zawh zel a, chuta tangin Khiangzawl kawnpui kawn tlaklam hawi zawnga luang a mawng atanga zawh chhovin, a a hoar kawn rekah a tan Iiarna. tichuan chhuahlamah ramri lui zawh thla in Tuirial a fin thla a, Tuirial elm a hnar lamah chhim hawi zawngin a zawh J..- chhova, Tuitial hna kak Darndiai lui a zawh chhova, Chawilung phul Leilet mawngah a chhuak chho a ui. Heta tang bian Jeep Road Lamchhip to Chawilnng kanin kawng ruh.hlui. zawh ..... chho zelin Ramrikawn chhak ta a han man a, chuta tangin chhuah Iamah Tui dum Iuiah a chhuk thla a, Sihpui lui a fin a, Sihpui lui chu khawchhak hawiin a zawh a, Mat lui a zuk fin a, Aizawl District Jeh Serchhip District inrina Mat lui chu ramri ah hmang nghalin Mat lui mawnglamah Tlangtla lui chhuah tbleng zawh thlain Tlanatla leh Mat infinna atangin khawthlang zawngin Tiangtla lui a bnar zawnga zawh chhovin Cnallui chhuah chhaka tlangdungin 111[- 305/2002 2 Tlangtla lui a pawh na thlengin a zawh chhova. -

Mizoram at a Glance 2018
STATE INFORMATION 1 MIZORAM : Mizoram is situated in the North Eastern part of India. It shares 404 km and 318 km long international borders with Myanmar and Bangladesh respectively. 2 Geographical Area : 21,081 Sq.Km. 3 Location : Between laltitude 21°.58' N to 24°.35' N and longitude 92°.15' E to 93°.29' E.The tropic of cancer runs through the state nearly at its middle. 4 State Capital : Aizawl. It is located in the northern part of Mizoram at an altitude of 1132 meters above sea level. 5 No. of District : 8. Namely- Mamit, Kolasib, Aizawl, Champhai, Serchhip, Lunglei, Lawngtlai, Siaha 6 No. of Assembly : 40 Constituencies 7 No. of Lok Sabha : 1 Seat 8 No. of Rajya Sabha : 1 Seat 9 State Animal : Saza (Serow) 10 State Bird : Vavu (Pheasant) 11 State Tree : Iron Wood (Herhse) 12 State Flower : Senhri (Red Vanda) 13 Highest Mountain : Phawngpui, in Siaha District, with an elevation of 2157 metres. 14 Longest River : Tlawng River, measuring 185.15 Km. It originates in Zopui Hill (Near Zobawk in Lunglei) at an elevation of 1,395 metres (4,577 ft). Page 1 STATE INFORMATION (Cont'd.) 15 Altitude of Tlabung : 21.03 Metre 16 Altitude of Murlen : 1877.30 Metre 17 Cimate of Aizawl : (i) Temperature during 2017 Average Max. - 28.6 C° Average Min. - 11.6 C˚ (ii) Humidity Average Max. - 93.5 % Average Min. - 88.0 % 18 Important Festival : Chapchar Kut, Christmas Festival, New Year Festival, Anthurium Festival, Lyuva Khutla Festival. 19 National Parks : Murlen National Park, Phawngpui National Park. 20 Important Wildlife : i) Animals: Serow, Hoolock Gibbon, Binturong, Indian Bison, Clouded Leopard ii) Birds: Hume's Pheasant, Great Indian Hornbill, Hornbill, Peacock Pheasant, Kalij Pheasant 21 Main Rivers : Tlawng, Tiau Lui, Chhimtuipui, Tut, Tuivai, Tuichang, Tuirial, Mat, Tuivawl, Tuirini, Serlui 22 Forest Area : 18748 km2 ( 88.93 % of the state geographical area) as per Forest Survey of India Report, 2015. -
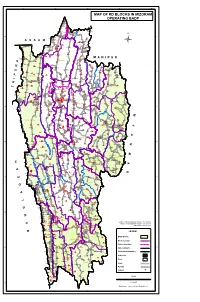
Map of Rd Blocks in Mizoram Operating Badp
92°20'0"E 92°40'0"E 93°60'0"E 93°20'0"E 93°40'0"E MAP OF RD BLOCKS IN MIZORAM Vairengte II OPERATING BADP Vairengte I Saihapui (V) Phainuam Chite Vakultui Saiphai Zokhawthiang North Chhimluang North Chawnpui Saipum Mauchar Phaisen Bilkhawthlir N 24°20'0"N 24°20'0"N Buhchang Bilkhawthlir S Chemphai North Thinglian Bukvannei I Tinghmun BuBkvIaLnKneHi IAI WTHLIR Parsenchhip Saihapui (K) Palsang Zohmun Builum Sakawrdai(Upper) Thinghlun(Lushaicherra) Hmaibiala Veng Rengtekawn Kanhmun South Chhimluang North Hlimen Khawpuar Lower Sakawrdai Luimawi KOLASIB N.Khawdungsei Vaitin Pangbalkawn Hriphaw Luakchhuah Thingsat Vervek E.Damdiai Bungthuam Bairabi New_Vervek Meidum North Thingdawl Thingthelh Lungsum Borai Saikhawthlir Rastali Dilzau H Thuampui(Zawlnuam) Suarhliap R Vengpuh i(Zawlnuam) i Chuhvel Sethawn a k DARLAWN g THINGDAWL Ratu n a Zamuang Kananthar L Bualpui Bukpui Zawlpui Damdiai Sunhluchhip Lungmawi Rengdil N.Khawlek Hortoki Sailutar Sihthiang R North Kawnpui I i R Daido a Vawngawnzo l Vanbawng v i Tlangkhang Kawnpui w u a T T v Mualvum North Chaltlang N.Serzawl i u u Chiahpui i N.E.Tlangnuam Khawkawn s T Darlawn a 24°60'0"N 24°60'0"N Lamherh R Kawrthah Khawlian Mimbung K Sarali North Sabual Sawleng Chilui Zanlawn N.E.Khawdungsei Saitlaw ZAWLNUAM Lungmuat Hrianghmun SuangpuilaPwnHULLEN Vengthar Tumpanglui Teikhang Venghlun Chhanchhuahna kepran Khamrang Tuidam Bazar Veng Nisapui MAMIT Phaizau Phuaibuang Liandophai(Bawngva) E.Phaileng Serkhan Luangpawn Mualkhang Darlak West Serzawl Pehlawn Zawngin Sotapa veng Sentlang T l Ngopa a Lungdai -

Mizoram Eco-Tourism Policy” As Enclosed
The Mizoram Gazette EXTRA ORDINARY Published by Authority RNI No. 27009/1973 Postal Regn. No. NE-313(MZ) 2006-2008 VOL - XLVI Aizawl, Friday 16.6.2017 Jyaistha 26, S.E. 1939, Issue No. 264 NOTIFICATION No. B. 12015/4/2016-FST, the 9th June, 2017. In order to provide recreation and promote understanding about heritage sites and its values, nature and wildlife conservation with income generation and employment opportunity to local people while according priority to bio-diversity conservation, ecosystem functioning and socio-economic development, the Governor of Mizoram is pleased to notify the “Mizoram Eco-Tourism Policy” as enclosed. Lalram Thanga, Principal Secretary to the Govt. of Mizoram, Environment, Forests & Climate Change Department. MIZORAM ECO-TOURISM POLICY 1 . Background: The state of Mizoram, endowed with numerous beautiful hill ranges covered with forest, rivers and streams between the hills, coupled with its rich natural and cultural heritage provides unique opportunities for ecotourism. Mizoram has about 88.93% of its land covered with forests (SFR 2015) that supports various species of mammals, birds, reptiles, more than 2500 species of plants including 253 species of orchids (BSI, Shillong) and more than 300 species of medicinal plants. The rich biodiversity of the state is conserved mainly through a network of protected areas comprising of 2 National Parks and 8 Sanctuaries complemented by wilderness areas, community conserved village forest areas, wetlands and other forest areas. Ecotourism or Ecologically Sustainable Tourism is defined as responsible travel to natural areas that conserves the environment and improves the well being of local communities. It is one of the tools whereby people are encouraged to visit natural forests to promote action to conserve it. -

DNA Barcoding Reveals a New Country Record for Three Species of Frogs (Amphibia: Anura) from India
Volume 20, issue 3, pages 106–117 30 September 2020 https://doi.org/10.33493/scivis.20.03.02 RESEARCH ARTICLE DNA barcoding reveals a new country record for three species of frogs (Amphibia: Anura) from India Samuel Lalronunga1,2*, Vanramliana3, C. Lalrinchhana4, Vanlalhrima2, Vanlalhriatzuala Sailo2, Lalnunhlua2, Lalkhawngaiha Sailo2, Isaac Zosangliana1,2, K. Lalhmangaiha1,2, Esther Lalhmingliani1,2 1Systematics and Toxicology Laboratory, Department of Zoology, Mizoram University, Aizawl 796004, Mizoram, India 2Biodiversity and Nature Conservation Network, Aizawl 796004, Mizoram, India 3Department of Zoology, Pachhunga University College, Aizawl 796001, Mizoram, India 4Holy Child School, Nalkata, Dhalai, Tripura 799263, India We present a first record for three species of frogs, viz. Amolops indoburmanensis, Received 25 May 2020 Accepted 12 August 2020 Euphlictys kalisgraminensis and Polypedates braueri in India based on DNA barcoding data. We also discussed on the records of Amolops spp. in Mizoram and *For correspondence: [email protected] delisted all previous recorded species, viz. A. afghanus, A. kaulbacki and A. marmoratus from the amphibian fauna of Mizoram until further research confirmed Contact us: [email protected] their presence, since the data were not sufficient enough for the confirmation of their occurrence and are likely based on misidentification of A. indoburmanensis. Key words: Biodiversity hotspot, conservation, 16S rRNA, northeast India, genetic distance. Introduction Four of the thirty six global biodiversity hotspots amphibian species identification and detection of partly fall within the territory of India, viz. Western cryptic species.11-14 Ghats, Indo-Burma, Himalaya and Sundaland.1-3 India Most part of northeast India falls within the Indo- harbours high species diversity and endemism in Burma and Himalaya biodiversity hotspots.