Movement Patterns of Pacific Crown-Of-Thorns Starfish (Acanthaster Cf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
W7192e19.Pdf
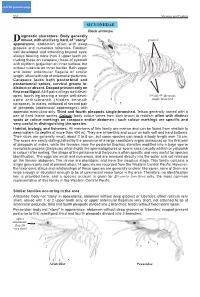
click for previous page 952 Shrimps and Prawns Sicyoniidae SICYONIIDAE Rock shrimps iagnostic characters: Body generally Drobust, with shell very hard, of “stony” grooves appearance; abdomen often with deep grooves and numerous tubercles. Rostrum well developed and extending beyond eyes, always bearing more than 3 upper teeth (in- cluding those on carapace); base of eyestalk with styliform projection on inner surface, but without tubercle on inner border. Both upper and lower antennular flagella of similar length, attached to tip of antennular peduncle. 1 Carapace lacks both postorbital and postantennal spines, cervical groove in- distinct or absent. Exopod present only on first maxilliped. All 5 pairs of legs well devel- 2 oped, fourth leg bearing a single well-devel- 3rd and 4th pleopods 4 single-branched oped arthrobranch (hidden beneath 3 carapace). In males, endopod of second pair 5 of pleopods (abdominal appendages) with appendix masculina only. Third and fourth pleopods single-branched. Telson generally armed with a pair of fixed lateral spines. Colour: body colour varies from dark brown to reddish; often with distinct spots or colour markings on carapace and/or abdomen - such colour markings are specific and very useful in distinguishing the species. Habitat, biology, and fisheries: All members of this family are marine and can be found from shallow to deep waters (to depths of more than 400 m). They are all benthic and occur on both soft and hard bottoms. Their sizes are generally small, about 2 to 8 cm, but some species can reach a body length over 15 cm. The sexes are easily distinguished by the presence of a large copulatory organ (petasma) on the first pair of pleopods of males, while the females have the posterior thoracic sternites modified into a large sperm receptacle process (thelycum) which holds the spermatophores or sperm sacs (usually whitish or yellowish in colour) after mating. -

Guam Crown-Of-Thorns Outbreak Response Plan December 2017 Guam COTS Outbreak Response Plan 1
Guam Crown-of-Thorns Outbreak Response Plan December 2017 Guam COTS Outbreak Response Plan 1 Overview The Guam Crown-of-Thorns sea star (COTS) Outbreak Response Plan was DevelopeD collaboratively by multiple local anD feDeral agencies, incluDing the Bureau of Statistics anD Plans (BSP) anD the Guam Coastal Management Program (GCMP), the Guam Department of Agriculture’s (GDOAG) Division of Aquatic anD WilDlife Resources (DAWR), the Guam Environmental Protection Agency (GEPA), the University of Guam Marine Laboratory (UOGML), the National Oceanic anD Atmospheric Association (NOAA), the National Park Service (NPS), Joint Region Marianas (JRM), anD the U.S. Fish & WilDlife Service (USFWS). The COTS Outbreak Response Plan exists to maximize effectiveness of activities conDucted by the Guam Coral Reef Response Team anD ensure efficient use of resources anD human capital by proviDing a stanDarDizeD framework for responDing to COTS outbreaks. This Document, designed as a working draft that will be continuously updated, includes an in-depth description of Guam’s early warning system for COTS outbreaks, stanDarD operating procedures for response implementation incluDing Detailed assessment anD mitigation protocols, anD recommenDations for post-outbreak management, reef recovery, and restoration approaches. This Document is intenDed for use by coral reef managers anD scientists on Guam, but may also be useful to inDividuals anD groups in other locations impacteD by COTS outbreaks, especially those who are interesteD in Developing COTS outbreak response plans. Objectives of the Guam COTS Response Plan: 1. Summarize impacts of past COTS outbreaks on Guam. 2. ProviDe up-to-date standard operating procedures to be followed before, during, and after COTS outbreaks, including lists of agency assets anD necessary supplies anD Delineation of agency roles. -

New Records of Marine Ornamental Shrimps (Decapoda: Stenopodidea and Caridea) from the Gulf of Mannar, Tamil Nadu, India
12 6 2010 the journal of biodiversity data 7 December 2016 Check List NOTES ON GEOGRAPHIC DISTRIBUTION Check List 12(6): 2010, 7 December 2016 doi: http://dx.doi.org/10.15560/12.6.2010 ISSN 1809-127X © 2016 Check List and Authors New records of marine ornamental shrimps (Decapoda: Stenopodidea and Caridea) from the Gulf of Mannar, Tamil Nadu, India Sanjeevi Prakash1, 3, Thipramalai Thangappan Ajith Kumar2* and Thanumalaya Subramoniam1 1 Centre for Climate Change Studies, Sathyabama University, Jeppiaar Nagar, Rajiv Gandhi Salai, Chennai - 600119, Tamil Nadu, India 2 ICAR - National Bureau of Fish Genetic Resources, Canal Ring Road, Dilkusha Post, Lucknow - 226002, Uttar Pradesh, India 3 Current address: Department of Biological Sciences, Clemson University, Clemson, SC 29634, USA * Corresponding author. E-mail: [email protected] Abstract: Marine ornamental shrimps found in from coral reefs have greatly affected their diversity and tropical coral reef waters are widely recognized for the distribution (Wabnitz et al. 2003). aquarium trade. Our survey of ornamental shrimps in Among all the ornamental shrimps, Stenopus the Gulf of Mannar, Tamil Nadu (India) has found three spp. and Lysmata spp. are the most attractive and species, which we identify as Stenopus hispidus Olivier, extensively traded organisms in the marine aquarium 1811, Lysmata debelius Bruce, 1983, and L. amboinensis industry (Calado 2008). Interestingly, these shrimps are De Man, 1888, based on morphology and color pattern. associates of fishes, in particular, the groupers and giant These shrimps are recorded for the first time in Gulf of moray eels (Gymnothorax spp.). These shrimps display a Mannar, Tamil Nadu. -

CORE Citation and Similar Papers at Core.Ac.Uk
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Aquatic Commons THE DEMONSTRATION OF CHEMOSENSORY FOOD DETECTION IN HYMENOCERA PICTA DANA (DECAPODA, CARIDEA), A PROPOSED PREDATOR OF THE CROWN-OF THORNS STARFISH ACANTHASTER PLANCI (L) PHILIP S. RAINBOW Cambridge Coral Starfish Research Group P.O. Box 99, Port Sudan, Democratic Republic of lhe Sudan ABSTRACT HymerlOcera pieta, the painted shrimp, is a possible predator of A canthaster planci. the Crown~of-Thorns slarfish. H. piela detects food by chemical cues alone and visual cues play no part in the initial location of prey. The presence of food in the water causes the shrimp to become more active, and distance chemoreceptors are probably present in the antennules of the shrimp. Extract of A. pfand has statistically similar attractive powers to an extract of Linckia multi/ora, the starfish supplied as food to the .<;hrimps. The painted shrimp was not attracted to fish extract (Chaetodon sp.) and may respond only to starfish. It is suggested that although H. pieta is able to kill and feed on small juvenile A. plan~i. it is probably an ineJTeclive predator against larger adult Crown·of-Thoms starfish. Present address: N.E.R.C. Unit. Department of Marine Science. U.C.N.W.• Menai Bridge, Anglesey. Wales. INTRODUCTION an Aean/haster individual, probabty as a Part of the research programme of the result of attack by the shrimps. From our Cambridge Coral Starfish Research Group own observations it seems probable that (ORMOND and CAMPBELL 1971; OR under natural conditions this shrimp would MOND et al. -

A New Record of Harlequin Shrimp (Malacostraca: Decapoda: Palaemonidae: Hymenocera Picta Dana, 1852) in the Southern
Journal of Threatened Taxa | www.threatenedtaxa.org | 26 August 2017 | 9(8): 10571–10576 A new record of Harlequin Shrimp (Malacostraca: Decapoda: Palaemonidae: Hymenocera picta Dana, 1852) in the southern Mexican Pacific Reefs ISSN 0974-7907 (Online) ISSN 0974-7893 (Print) Communication Short Omar Valencia-Mendez 1, Andres Lopez-Perez 2, Betel Martinez-Guerrero 3, 4 5 Virgilio Antonio-Perez & Eduardo Ramirez-Chavez OPEN ACCESS 1 Programa de Doctorado en Ciencias Biológicas y de la Salud, 2 Departamento de Hidrobiología, Universidad Autónoma Metropolitana, San Rafael Atlixco 186, Col. Vicentina CP09340, Ciudad de México, México 3,5 Universidad del Mar, Campus Puerto Ángel, Carretera a Zipolite Km. 1.5, Col. Puerto Ángel, San Pedro Pochutla, Oaxaca, 70902, México 4 Buceo Huatulco, Ocotillo # 206, La Crucecita, Bahías de Huatulco, Oaxaca, 70989, México 1 [email protected], 2 [email protected] (corresponding author), 3 [email protected], 4 [email protected], 5 [email protected] Abstract: The Harlequin Shrimp Hymenocera picta is abundant in the Hymenocera is a small decapod crustacean (~5cm Indo-Pacific and Central Pacific regions, but there are few reports of in TL) belonging to the superfamily Palaemonoidea it from the eastern Pacific. Two pairs of the Harlequin Shrimp were observed feeding on the Sea Star Phataria unifascialis (Gray, 1840) Rafinesque, 1815 sensu De Grave & Fransen (2011). Due in the reefs of Huatulco National Park, Mexican Pacific. This paper to its amazing coloration, it is one of the most in-demand reports the occurrence of H. picta in Mexican Pacific waters and extends its previous distribution by 1,270km north of El Ocotal, Costa decapod crustacean species in the marine ornamental Rica in the eastern Pacific equatorial zone. -

© the Authors 2019. All Rights Reserved
© The Authors 2019. All rights reserved. www.publish.csiro.au Index Note: Bold page numbers refer to illustrations. abalones 348 tenella 77 Acanthaster 134, 365, 367, 393 tenuis 272 mauritiensis 134, 135 white syndrome 152 planci 134 Acroporidae 136, 274–5, 276 solaris 367 Acrozoanthus australiae 264, 265 cf. solaris 134, 135, 144, 165, 270, 275, 339, 345, 366 acrozooid polyps 287 sp. A nomen nudum 134 Actaeomorpha 337 Acanthaster outbreaks 134–5 scruposa 335 causes 134–5, 144–6, 163–4, 165, 367 Acteonidae 349 management 135 Actinaria 257, 259–63 see also crown-of-thorns starfish (COTS) outbreaks anatomical features 258 Acanthasteridae 367 cnidae types in 260 Acanthastrea echinata 279 Actiniidae 263 Acanthella cavernosa 236, 238 Actinocyclidae 349 Acanthochitonidae 349 Actinodendron glomeratum 261 Acanthogorgia 302, 306 Actinopyga 369, 370 sp. 302 echinites 372 Acanthogorgiidae 302 miliaris 372 Acanthopargus spp. 126 sp. 372 Acanthopleura gemmata 107, 110 Aegiceras corniculatum 221, 222, 223 Acanthuridae 390, 396 Aegiridae 349 Acanthurus aeolid nudibranchs 346, 347, 349 blochii 396 Aeolidiidae 349 lineatus 395, 396 Aequorea 202 mata 393 Afrocucumis africana 368, 370 olivaceus 395 Agariciidae 275, 276 Acartia sp. 192 Agelas axifera 236, 238 accessory pigments 89 aggressive mimicry 401 Acetes 333 Agjajidae 349 sp. 192 agricultural activities, sediments and nutrients from 161–5 Achelata 333, 334–6 Ailsastra sp. 368 acorn barnacles 328 Aiptasia pulchella 260 acorn dog whelk 346, 347 Aipysurus 415, 416 Acropora 31, 33, 59, 135, 136, 150, 184, 211, 272, 273, 274–5, 339 duboisii 412, 413, 415 brown band disease 152 laevis 412, 413, 415, 416 clathrata 79 mosaicus 415 echinata 276 Alcyonacea 67, 283, 290–309 global diversity 186 Alcyonidium sp. -

Sense of Smell and Pair• Bond in Hymenocera Picta Dana 1
Sense of Smell and Pair• Bond in Hymenocera picta Dana 1 UTA SEIBT 2 Abstract Hymenocera picta Dana has function al eyes and is able to recognize conspecifics b sight over 20 cm away. Prey, however, is identified by smell and located by the shrimp yovingupstream as long as the scent is perceived. Scents are perceived by the waving :Otennuleswhich have a single row of sensory hairs on their terminal lobes . Hym enocera are normally found in pairs: 93.5 per cent heterosexual and 6.5 per cent homosexual. Therefore, both sexes must be able to identify the sex of a conspecific. Using only scent cues, theshrimp does prefer conspecifics over other crustacea. Males identify by scent only and prefer their own female over any other conspecific. A pheromone is produced only by the female and perceived by males only . Motivation analysis shows that the pair bond (the attachment to a particular individual of opposite sex) in Hymen ocera is not part of the agonistic, or sexual, or brood-care behavior complex, but is based on a special "drive for attachment". We suggest that attachment to a conspecific in Hymeno cera is a means of reducing stress in the individual. In a preceding paper, the general biology of Hymenocera picta Dana has been described, including the fact that these animals are found in pairs (Wickler, 1973- thisissue). Over the period of one year, we put to protocol all the pairings among 65animals (28 females and 37 males) . From a total of 1352 pairings, 95.5 per cent wereheterosexual, 3.3 per cent were male-pairings, and 3.2 per cent were female pairings. -

Other Crustaceans Unauna Or Hermit Crabs Crabs Shrimps
Marine Invertebrates Other Crustaceans Unauna or Hermit crabs Aniculus hopperae Calcinus hazletti Calcinus laurentae Crabs Aethra edentate Carpilius maculatus Dromia dromia Ligia hawaiensis Lybia edmondsoni Pseudopalicus oahuensis Shrimps Cinetorhynchus hawaiiensis Cinetorhynchus hendersoni Gnathophyllum precipuum Hymenocera picta Levicaris mammilata Liomera supernodosa Metapenaeopsis sp. Rhynchocinetes rathbunae Stenopus earlei SPECIES STATUS: IUCN Red List - Not considered All Endemic except for Carpilius, Dromia, and Hymenocera SPECIES INFORMATION: The following are the Hawaiian, common, and scientific names for the Unauna or hermit crabs, true crabs, and shrimps: Hopper’s hermit crab (Aniculus hopperae), Hazlett’s hermit crab (Calcinus hazletti) and Laurent’s hermit crab (Calcinus laurentae); flat elbow Hawai’i’s State Wildlife Action Plan October 1, 2015 (Last Updated October 2005) crab (Aethra edentata), alakuma or 7-11 crab (Carpilius maculates), makua-o-ka-lipoa or sponge crab (Dromia dormia), Ligia hawaiensis (no common name), kūmimi pua or Hawaiian pom pom crab or (Lybia edmondsoni), and button crab (Pseudopalicus oahuensis); Hawaiian hinge-beaked shrimp (Cinetorhynchus hawaiiensis), Henderson’s hinge-beaked shrimp (Cinetorhynchus hendersoni), Hawaiian cave shrimp (Gnathophyllum precipuum), harlequin shrimp (Hymenocera picta), red pencil urchin shrimp (Levicaris mammilata), knotted liomera (Liomera supernodosa), bicolor sand shrimp (Metanpenaeopsis sp.), Rathbun’s hinge-beaked shrimp (Rhynchocinetes rathbunae), and Earl’s coral shrimp (Stenopus earlei). The unauna, alakuma, button crab, hinge- beaked shrimp, Hawaiian cave shrimp, and the bicolor sand shrimp are nocturnal. Hermit crabs are scavengers, Earl’s coral shrimp are cleaners, alakuma crush other crustaceans and snails, button crabs feed on algae, and kūmimi pua use anemones on their claws to capture prey and feed on invertebrates. -
Elena Tricarico Editors Social Recognition in Invertebrates the Knowns and the Unknowns Social Recognition in Invertebrates Laura Aquiloni · Elena Tricarico Editors
Laura Aquiloni · Elena Tricarico Editors Social Recognition in Invertebrates The Knowns and the Unknowns Social Recognition in Invertebrates Laura Aquiloni · Elena Tricarico Editors Social Recognition in Invertebrates The Knowns and the Unknowns 1 3 Editors Laura Aquiloni Elena Tricarico Department of Biology Department of Biology University of Florence University of Florence Florence Florence Italy Italy ISBN 978-3-319-17598-0 ISBN 978-3-319-17599-7 (eBook) DOI 10.1007/978-3-319-17599-7 Library of Congress Control Number: 2015936295 Springer Cham Heidelberg New York Dordrecht London © Springer International Publishing Switzerland 2015 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. The publisher, the authors and the editors are safe to assume that the advice and information in this book are believed to be true and accurate at the date of publication. Neither the publisher nor the authors or the editors give a warranty, express or implied, with respect to the material contained herein or for any errors or omissions that may have been made. -
F F F F . F F F F F F F F F F F F F F 3 F F F F F F F F F F F F F F F F F
1 . !" #$% "&' ()( " * * + ,- &. " /&" & !" 0 1 2&# # # 311# # 4#5 &+6 1 & 3 8 2* & & *+ 22 " 1 ##'1 & *+) 2 2 %''&$* 8 25 & *+ " / " " 2 % &+ 22 1 & !2" &+ 1 & 1 #'1 25 & *+ 22" / # 2 1 , 1 ##'1 */ 8 25 & * + 22" / , 1 #* % % %''& $*25 %''&$*" / 21 # 8 ;<= ;2 *1- '/ >5* + 1(!# 1 "? */ 25' '. " )(%-% *@ 2 8 "" ;3 % # "? , 8@& !, &% ,, %-% 6841 2 A' , 0 & . ' ( 2 + , Kingdom Animalia, Phylum Arthropoda, Class Malacostraca, Order Decapoda, Family Hymenoceridae, Genus Hymenocera '/ /&6 " Hymenocera picta Dana, 1852 /* Hymenocera elegans Heller, 1861 / " Harlequin shrimp, Painted shrimp, Clown shrimp, Dancing shrimp, Harley Shrimp 2 0 * &" , &"5 Hymenocera 1 26 Hymenoceridae 1 . [] : http://th.wikipedia.org/wiki ,-. /- 15 . 2555 2 0 * + ,- %.&*#5 &2 5 '& 2 '&2);d 2#" 0 * + 2 3 !2" picta *#$%2$% 2);d& 1(*" '/ # #, 2 elegans *#'& 22);d )( 0 1 ,- (, 2 #;e. - 1-2 , '/ 2-5 ) *#& #%( 3-60 ; (1-18 )4 k 2-5 l5 - , 2 .2/ !!"" .& - @ 5 ) . 1%#&? 18-26 / %# 1 !* + 2 ! " 1! "!&' &! "1! "& " ,- ! "% , 100-5,000 ; (, " #2 2"* + ! " * 0 " 2"1&5 17-24 ") , (zoea) ,- 2##2*% %/"! 2 ,- 8@'' 2 6 %#'% , " 5 28 - 56 */ 2"1 ) , (zoea) 0 * (postlarva) '/ */, (%-) 5 ,1 !"&%2 .5 " !% (Echinoderms) !2" " & (sea urchin) (brittle sea stars) 2& (sea star) 0 ' 1 22& */ /, 5 8@ " 1 # - ,' (, 21*#&%' #) 0 r &12 1(! # " s#)2'"&& s '/ t%" 2'" s #" 0 %"? 1 , /2 . 2 1;e ,- / %" !& '/ 2 "!"1 ,- " 11 ,- 1 &2 " 5 2 "u 6 - '& Indo-west Pacific 12 & ;e'/ ,- # @ # - Central-Eastern Pacific k*& y12 & "'/" 2 & ,2'" '/ '/'/ ,- "$5 12 ,- '/ ,- &2# @" ' ($*& 1) 2 Sammy De Grave (2010). "Hymenocera Latreille, 1819" . World Register of Marine Species . http://www.marinespecies.org/aphia.php?p=taxdetails&id=204637 . -

Grooming Behavior and Morphology of the Caridean Shrimp Pandalus Danae Stimpson (Decapoda: Natantia: Pandalidae)
January 1975 Grooming behavior and morphology of the caridean shrimp Pandalus danae Stimpson (Decapoda: Natantia: Pandalidae) RAYMOND T. BAUER Scripps Institution of Oceanography, LaJolla, California U.S.A. Accepted for publication July 1974 brushes on thoracic limbs scrape and rub other appendages and general surfaces of the exoskeleton. Chelate limbs nip and pick at edges of crevices in the cephalothoracic region. Scanning electron microscopy reveals that the setae composing the grooming brushes are equipped with characteristic tooth- and scale-like setules which serve as the actual rasping devices. Antennules. antennae and pereopods are frequently preened by the third maxillipeds and first pereopods, while large areas of the exoskeleton are cleaned by the third pair of walking legs and the chelate limbs. Shrimp with the general cleaning limbs ablated develop significantly greater infestations of the epizoic suctorian Ephelota than animals allowed to groom. Olfactory hairs on the antennules of shrimp deprived of the third maxillipeds become fouled with diatoms and debris while those on controls do not. Grooming behavior clearly prevents a build-up of settling organisms between molts and repeated cleaning of sensory sites is essential in maintaining contact with the environment. CONTENTS Introduction 45 Materials and methods +7 Morphology and behavior *7 Third maxillipeds +8 First pereopods 5 3 Second pereopods 56 Fifth pereopods 59 Experiments 62 Results 62 General grooming experiment 62 Antennular grooming experiment 65 Discussion 66 Acknowledgements 68 References 68 Addendum 69 INTRODUCTION A characteristic of the marine environment is the abundance of the larvae and spores of sessile organisms and the limited amount of suitable surface for 4S 46 R. -

The First Mitochondrial Genome of the Genus Exhippolysmata (Decapoda
www.nature.com/scientificreports OPEN The frst mitochondrial genome of the genus Exhippolysmata (Decapoda: Caridea: Lysmatidae), with gene rearrangements and phylogenetic associations in Caridea Ying‑ying Ye1,2,3*, Jing Miao2, Ya‑hong Guo2, Li Gong2,3, Li‑hua Jiang2,3, Zhen‑ming Lü2,3, Kai‑da Xu1* & Bao‑ying Guo2,3 The complete mitochondrial genome (mitogenome) of animals can provide useful information for evolutionary and phylogenetic analyses. The mitogenome of the genus Exhippolysmata (i.e., Exhippolysmata ensirostris) was sequenced and annotated for the frst time, its phylogenetic relationship with selected members from the infraorder Caridea was investigated. The 16,350 bp mitogenome contains the entire set of 37 common genes. The mitogenome composition was highly A + T biased at 64.43% with positive AT skew (0.009) and negative GC skew (− 0.199). All tRNA genes in the E. ensirostris mitogenome had a typical cloverleaf secondary structure, except for trnS1 (AGN), which appeared to lack the dihydrouridine arm. The gene order in the E. ensirostris mitogenome was rearranged compared with those of ancestral decapod taxa, the gene order of trnL2‑cox2 changed to cox2‑trnL2. The tandem duplication‑random loss model is the most likely mechanism for the observed gene rearrangement of E. ensirostris. The ML and BI phylogenetic analyses place all Caridea species into one group with strong bootstrap support. The family Lysmatidae is most closely related to Alpheidae and Palaemonidae. These results will help to better understand the gene rearrangements and evolutionary position of E. ensirostris and lay a foundation for further phylogenetic studies of Caridea. Te Decapoda is an ecologically and economically important order of crustaceans comprising a wide variety of crabs, lobsters, prawns and shrimps totalling over 18,000 extant and fossil species1,2.