Social Behaviour and Recognition in Decapod Shrimps, with Emphasis on the Caridea
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Two Freshwater Shrimp Species of the Genus Caridina (Decapoda, Caridea, Atyidae) from Dawanshan Island, Guangdong, China, with the Description of a New Species
A peer-reviewed open-access journal ZooKeys 923: 15–32 (2020) Caridina tetrazona 15 doi: 10.3897/zookeys.923.48593 RESEarcH articLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Two freshwater shrimp species of the genus Caridina (Decapoda, Caridea, Atyidae) from Dawanshan Island, Guangdong, China, with the description of a new species Qing-Hua Chen1, Wen-Jian Chen2, Xiao-Zhuang Zheng2, Zhao-Liang Guo2 1 South China Institute of Environmental Sciences, Ministry of Ecology and Environment, Guangzhou 510520, Guangdong Province, China 2 Department of Animal Science, School of Life Science and Enginee- ring, Foshan University, Foshan 528231, Guangdong Province, China Corresponding author: Zhao-Liang Guo ([email protected]) Academic editor: I.S. Wehrtmann | Received 19 November 2019 | Accepted 7 February 2020 | Published 1 April 2020 http://zoobank.org/138A88CC-DF41-437A-BA1A-CB93E3E36D62 Citation: Chen Q-H, Chen W-J, Zheng X-Z, Guo Z-L (2020) Two freshwater shrimp species of the genus Caridina (Decapoda, Caridea, Atyidae) from Dawanshan Island, Guangdong, China, with the description of a new species. ZooKeys 923: 15–32. https://doi.org/10.3897/zookeys.923.48593 Abstract A faunistic and ecological survey was conducted to document the diversity of freshwater atyid shrimps of Dawanshan Island. Two species of Caridina that occur on this island were documented and discussed. One of these, Caridina tetrazona sp. nov. is described and illustrated as new to science. It can be easily distinguished from its congeners based on a combination of characters, which includes a short rostrum, the shape of the endopod of the male first pleopod, the segmental ratios of antennular peduncle and third maxilliped, the slender scaphocerite, and the absence of a median projection on the posterior margin. -

Decapod Crustacean Grooming: Functional Morphology, Adaptive Value, and Phylogenetic Significance
Decapod crustacean grooming: Functional morphology, adaptive value, and phylogenetic significance N RAYMOND T.BAUER Center for Crustacean Research, University of Southwestern Louisiana, USA ABSTRACT Grooming behavior is well developed in many decapod crustaceans. Antennular grooming by the third maxillipedes is found throughout the Decapoda. Gill cleaning mechanisms are qaite variable: chelipede brushes, setiferous epipods, epipod-setobranch systems. However, microstructure of gill cleaning setae, which are equipped with digitate scale setules, is quite conservative. General body grooming, performed by serrate setal brushes on chelipedes and/or posterior pereiopods, is best developed in decapods at a natant grade of body morphology. Brachyuran crabs exhibit less body grooming and virtually no specialized body grooming structures. It is hypothesized that the fouling pressures for body grooming are more severe in natant than in replant decapods. Epizoic fouling, particularly microbial fouling, and sediment fouling have been shown r I m ans of amputation experiments to produce severe effects on olfactory hairs, gills, and i.icubated embryos within short lime periods. Grooming has been strongly suggested as an important factor in the coevolution of a rhizocephalan parasite and its anomuran host. The behavioral organization of grooming is poorly studied; the nature of stimuli promoting grooming is not understood. Grooming characters may contribute to an understanding of certain aspects of decapod phylogeny. The occurrence of specialized antennal grooming brushes in the Stenopodidea, Caridea, and Dendrobranchiata is probably not due to convergence; alternative hypotheses are proposed to explain the distribution of this grooming character. Gill cleaning and general body grooming characters support a thalassinidean origin of the Anomura; the hypothesis of brachyuran monophyly is supported by the conservative and unique gill-cleaning method of the group. -
W7192e19.Pdf
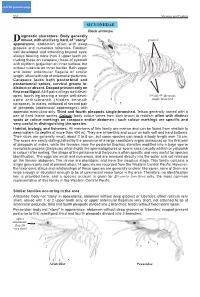
click for previous page 952 Shrimps and Prawns Sicyoniidae SICYONIIDAE Rock shrimps iagnostic characters: Body generally Drobust, with shell very hard, of “stony” grooves appearance; abdomen often with deep grooves and numerous tubercles. Rostrum well developed and extending beyond eyes, always bearing more than 3 upper teeth (in- cluding those on carapace); base of eyestalk with styliform projection on inner surface, but without tubercle on inner border. Both upper and lower antennular flagella of similar length, attached to tip of antennular peduncle. 1 Carapace lacks both postorbital and postantennal spines, cervical groove in- distinct or absent. Exopod present only on first maxilliped. All 5 pairs of legs well devel- 2 oped, fourth leg bearing a single well-devel- 3rd and 4th pleopods 4 single-branched oped arthrobranch (hidden beneath 3 carapace). In males, endopod of second pair 5 of pleopods (abdominal appendages) with appendix masculina only. Third and fourth pleopods single-branched. Telson generally armed with a pair of fixed lateral spines. Colour: body colour varies from dark brown to reddish; often with distinct spots or colour markings on carapace and/or abdomen - such colour markings are specific and very useful in distinguishing the species. Habitat, biology, and fisheries: All members of this family are marine and can be found from shallow to deep waters (to depths of more than 400 m). They are all benthic and occur on both soft and hard bottoms. Their sizes are generally small, about 2 to 8 cm, but some species can reach a body length over 15 cm. The sexes are easily distinguished by the presence of a large copulatory organ (petasma) on the first pair of pleopods of males, while the females have the posterior thoracic sternites modified into a large sperm receptacle process (thelycum) which holds the spermatophores or sperm sacs (usually whitish or yellowish in colour) after mating. -

Caribbean Wildlife Undersea 2017
Caribbean Wildlife Undersea life This document is a compilation of wildlife pictures from The Caribbean, taken from holidays and cruise visits. Species identification can be frustratingly difficult and our conclusions must be checked via whatever other resources are available. We hope this publication may help others having similar problems. While every effort has been taken to ensure the accuracy of the information in this document, the authors cannot be held re- sponsible for any errors. Copyright © John and Diana Manning, 2017 1 Angelfishes (Pomacanthidae) Corals (Cnidaria, Anthozoa) French angelfish 7 Bipinnate sea plume 19 (Pomacanthus pardu) (Antillogorgia bipinnata) Grey angelfish 8 Black sea rod 20 (Pomacanthus arcuatus) (Plexaura homomalla) Queen angelfish 8 Blade fire coral 20 (Holacanthus ciliaris) (Millepora complanata) Rock beauty 9 Branching fire coral 21 (Holacanthus tricolor) (Millepora alcicornis) Townsend angelfish 9 Bristle Coral 21 (Hybrid) (Galaxea fascicularis) Elkhorn coral 22 Barracudas (Sphyraenidae) (Acropora palmata) Great barracuda 10 Finger coral 22 (Sphyraena barracuda) (Porites porites) Fire coral 23 Basslets (Grammatidae) (Millepora dichotoma) Fairy basslet 10 Great star coral 23 (Gramma loreto) (Montastraea cavernosa) Grooved brain coral 24 Bonnetmouths (Inermiidae) (Diploria labyrinthiformis) Boga( Inermia Vittata) 11 Massive starlet coral 24 (Siderastrea siderea) Bigeyes (Priacanthidae) Pillar coral 25 Glasseye snapper 11 (Dendrogyra cylindrus) (Heteropriacanthus cruentatus) Porous sea rod 25 (Pseudoplexaura -

Guam Crown-Of-Thorns Outbreak Response Plan December 2017 Guam COTS Outbreak Response Plan 1
Guam Crown-of-Thorns Outbreak Response Plan December 2017 Guam COTS Outbreak Response Plan 1 Overview The Guam Crown-of-Thorns sea star (COTS) Outbreak Response Plan was DevelopeD collaboratively by multiple local anD feDeral agencies, incluDing the Bureau of Statistics anD Plans (BSP) anD the Guam Coastal Management Program (GCMP), the Guam Department of Agriculture’s (GDOAG) Division of Aquatic anD WilDlife Resources (DAWR), the Guam Environmental Protection Agency (GEPA), the University of Guam Marine Laboratory (UOGML), the National Oceanic anD Atmospheric Association (NOAA), the National Park Service (NPS), Joint Region Marianas (JRM), anD the U.S. Fish & WilDlife Service (USFWS). The COTS Outbreak Response Plan exists to maximize effectiveness of activities conDucted by the Guam Coral Reef Response Team anD ensure efficient use of resources anD human capital by proviDing a stanDarDizeD framework for responDing to COTS outbreaks. This Document, designed as a working draft that will be continuously updated, includes an in-depth description of Guam’s early warning system for COTS outbreaks, stanDarD operating procedures for response implementation incluDing Detailed assessment anD mitigation protocols, anD recommenDations for post-outbreak management, reef recovery, and restoration approaches. This Document is intenDed for use by coral reef managers anD scientists on Guam, but may also be useful to inDividuals anD groups in other locations impacteD by COTS outbreaks, especially those who are interesteD in Developing COTS outbreak response plans. Objectives of the Guam COTS Response Plan: 1. Summarize impacts of past COTS outbreaks on Guam. 2. ProviDe up-to-date standard operating procedures to be followed before, during, and after COTS outbreaks, including lists of agency assets anD necessary supplies anD Delineation of agency roles. -

A New Classification of the Xanthoidea Sensu Lato
Contributions to Zoology, 75 (1/2) 23-73 (2006) A new classifi cation of the Xanthoidea sensu lato (Crustacea: Decapoda: Brachyura) based on phylogenetic analysis and traditional systematics and evaluation of all fossil Xanthoidea sensu lato Hiroaki Karasawa1, Carrie E. Schweitzer2 1Mizunami Fossil Museum, Yamanouchi, Akeyo, Mizunami, Gifu 509-6132, Japan, e-mail: GHA06103@nifty. com; 2Department of Geology, Kent State University Stark Campus, 6000 Frank Ave. NW, North Canton, Ohio 44720, USA, e-mail: [email protected] Key words: Crustacea, Decapoda, Brachyura, Xanthoidea, Portunidae, systematics, phylogeny Abstract Family Pilumnidae ............................................................. 47 Family Pseudorhombilidae ............................................... 49 A phylogenetic analysis was conducted including representatives Family Trapeziidae ............................................................. 49 from all recognized extant and extinct families of the Xanthoidea Family Xanthidae ............................................................... 50 sensu lato, resulting in one new family, Hypothalassiidae. Four Superfamily Xanthoidea incertae sedis ............................... 50 xanthoid families are elevated to superfamily status, resulting in Superfamily Eriphioidea ......................................................... 51 Carpilioidea, Pilumnoidoidea, Eriphioidea, Progeryonoidea, and Family Platyxanthidae ....................................................... 52 Goneplacoidea, and numerous subfamilies are elevated -

Prawn Fauna (Crustacea: Decapoda) of India - an Annotated Checklist of the Penaeoid, Sergestoid, Stenopodid and Caridean Prawns
Available online at: www.mbai.org.in doi: 10.6024/jmbai.2012.54.1.01697-08 Prawn fauna (Crustacea: Decapoda) of India - An annotated checklist of the Penaeoid, Sergestoid, Stenopodid and Caridean prawns E. V. Radhakrishnan*1, V. D. Deshmukh2, G. Maheswarudu3, Jose Josileen 1, A. P. Dineshbabu4, K. K. Philipose5, P. T. Sarada6, S. Lakshmi Pillai1, K. N. Saleela7, Rekhadevi Chakraborty1, Gyanaranjan Dash8, C.K. Sajeev1, P. Thirumilu9, B. Sridhara4, Y Muniyappa4, A.D.Sawant2, Narayan G Vaidya5, R. Dias Johny2, J. B. Verma3, P.K.Baby1, C. Unnikrishnan7, 10 11 11 1 7 N. P. Ramachandran , A. Vairamani , A. Palanichamy , M. Radhakrishnan and B. Raju 1CMFRI HQ, Cochin, 2Mumbai RC of CMFRI, 3Visakhapatnam RC of CMFRI, 4Mangalore RC of CMFRI, 5Karwar RC of CMFRI, 6Tuticorin RC of CMFRI, 7Vizhinjam RC of CMFRI, 8Veraval RC of CMFRI, 9Madras RC of CMFRI, 10Calicut RC of CMFRI, 11Mandapam RC of CMFRI *Correspondence e-mail: [email protected] Received: 07 Sep 2011, Accepted: 15 Mar 2012, Published: 30 Apr 2012 Original Article Abstract Many penaeoid prawns are of considerable value for the fishing Introduction industry and aquaculture operations. The annual estimated average landing of prawns from the fishery in India was 3.98 The prawn fauna inhabiting the marine, estuarine and lakh tonnes (2008-10) of which 60% were contributed by freshwater ecosystems of India are diverse and fairly well penaeid prawns. An additional 1.5 lakh tonnes is produced from known. Significant contributions to systematics of marine aquaculture. During 2010-11, India exported US $ 2.8 billion worth marine products, of which shrimp contributed 3.09% in prawns of Indian region were that of Milne Edwards (1837), volume and 69.5% in value of the total export. -

Antifouling Adaptations of Marine Shrimp (Decapoda: Caridea): Gill Cleaning Mechanisms and Grooming of Brooded Embryos
Zoological Journal oj the Linnean Society, 6i: 281-305. With 12 figures April 1979 Antifouling adaptations of marine shrimp (Decapoda: Caridea): gill cleaning mechanisms and grooming of brooded embryos RAYMOND T. BAUER Biological Sciences, California Polytechnic State University, San Luis Obispo, California, U.S.A. Accepted for publication September 1977 Gills in the branchial chambers of caridean shrimps, as well as the brooded embryos in females, are subject to fouling by particulate debris and epizoites. Important mechanisms for cleaning the gills are brushing of the gills by the grooming or cleaning chelipeds in some species, while in others, setae from the bases of the thoracic legs brush up among the gills during movement of the limbs (epipod- setobranch complexes). Setae of cleaning chelipeds and of epipod-setobranch complexes show- similar ultrastructural adaptions for scraping gill surfaces. Ablation of the cleaning chelipeds ol the shrimp Heptacarpm pictus results in severe fouling of the gills in experimenials, while those of controls remain clean, Embrvos brooded by female carideans are often brushed and jostled by the grooming chelipeds. In H. pictui. removal of the cleaning chelae results in heavier microbial and sediment fouling than in controls. KEY WO RDS: - shrimp - gills - grooming - cleaning - cpipods - sctobranchs - fouling - Decapoda - Caridea. CONTENTS Introduction 281 Methods 282 Results 284 Gill cleaning by the chelipeds 284 Gill cleaning by the epipod-setobranch complex 289 Experiments on the adaptive value of cheliped brushing in Heptacarpus pictus 292 Cleaning of brooded embryos 296 Experiments on the adaptive value of cheliped brushing of eggs in Heptacarpus pictus 297 Discussion 299 Adaptive value of gill cleaning mechanisms in caridean shrimp 299 Adaptive value of embryo brushing by females 301 Acknowledgements 302 References 302 INTRODUCTION Grooming behaviour is a frequent activity of caridean shrimp which appears to prevent epizoic and sediment fouling of the body (Bauer, 1975; Bauer, 1977, Bauer, 1978). -

<I>Bartholomea Annulata</I>
BULLETIN OF MARINE SCIENCE, 37(3): 893-904,1985 CORAL REEF PAPER TWO MORE SIBLING SPECIES OF ALPHEID SHRIMPS ASSOCIATED WITH THE CARIBBEAN SEA ANEMONES BARTHOLOMEA ANNULATA AND HETERACTIS LUCIDA Nancy Knowlton and Brian D. Keller ABSTRACT We have described two new species of snapping shrimp, Alpheus polystictus and A. ro- quensis. The new species form part of a complex of four sibling species associated with Caribbean sea anemones, the others being the well-known A. armatus Rathbun, 1900 and the recently describedA. immaculatus Knowlton and Keller, 1983. Alpheus roquensis is found with the anemone Heteractis lucida. while the other three shrimps live with Bartholomea annulata. In laboratory choice experiments, each shrimp species prefers the species of an em- one with which it is typically found in the field, although each can shelter under the other species of anemone. All four species are extremely similar morphologically, being distin- guished largely on the basis of color pattern. The validity of the species is confirmed by the total absence of interbreeding; heterospecific male-female pairs are never found in the field, and it is impossible to force pairings between species in the laboratory. Alpheus polystictus is rare in Jamaica and Haiti, while in Venezuela it is sometimes the dominant species to depths of 10 m. In the areas examined, it has always occurred with at least one of the other two Bartholomea associates. The geographic distribution of A. roquensis is more limited, as there are no reports of alpheids associated with Heteractis lucida, and none has been found with this anemone in Jamaica. -

Epibenthic Mobile Invertebrates Along the Florida Reef Tract: Diversity and Community Structure Kristin Netchy University of South Florida, [email protected]
University of South Florida Scholar Commons Graduate Theses and Dissertations Graduate School 3-21-2014 Epibenthic Mobile Invertebrates along the Florida Reef Tract: Diversity and Community Structure Kristin Netchy University of South Florida, [email protected] Follow this and additional works at: https://scholarcommons.usf.edu/etd Part of the Ecology and Evolutionary Biology Commons, Other Education Commons, and the Other Oceanography and Atmospheric Sciences and Meteorology Commons Scholar Commons Citation Netchy, Kristin, "Epibenthic Mobile Invertebrates along the Florida Reef Tract: Diversity and Community Structure" (2014). Graduate Theses and Dissertations. https://scholarcommons.usf.edu/etd/5085 This Thesis is brought to you for free and open access by the Graduate School at Scholar Commons. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. Epibenthic Mobile Invertebrates along the Florida Reef Tract: Diversity and Community Structure by Kristin H. Netchy A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science Department of Marine Science College of Marine Science University of South Florida Major Professor: Pamela Hallock Muller, Ph.D. Kendra L. Daly, Ph.D. Kathleen S. Lunz, Ph.D. Date of Approval: March 21, 2014 Keywords: Echinodermata, Mollusca, Arthropoda, guilds, coral, survey Copyright © 2014, Kristin H. Netchy DEDICATION This thesis is dedicated to Dr. Gustav Paulay, whom I was fortunate enough to meet as an undergraduate. He has not only been an inspiration to me for over ten years, but he was the first to believe in me, trust me, and encourage me. -

Protection of Host Anemones by Snapping Shrimps: a Case for Symbiotic Mutualism?
Symbiosis DOI 10.1007/s13199-014-0289-8 Protection of host anemones by snapping shrimps: a case for symbiotic mutualism? AmberM.McCammon& W. Randy Brooks Received: 4 June 2014 /Accepted: 29 July 2014 # Springer Science+Business Media Dordrecht 2014 Abstract The sea anemone Bartholomea annulata is an eco- especially common in marine environments (Roughgarden logically important member of Caribbean coral reefs which host 1975; Poulin and Grutter 1996;Côté2000). Mutualism; a a variety of symbiotic crustacean associates. Crustacean type of symbiotic relationship in which both partners derive exosymbionts typically gain protection from predation by dwell- some benefit from the association, are also widespread across ing with anemones. Concurrently, some symbionts may provide taxa (Boucher et al. 1982). The benefit(s) of symbiont- protection to their host by defending against anemone predators mediated protection of host species from microbial disease, such as the predatory fireworm, Hermodice carunculata,which parasites, and predators is increasingly evident (Haine 2008). can severely damage or completely devour prey anemones. Protection mechanisms are diverse and include various sym- Herein we show through both field and laboratory studies that biont derived chemical defenses (Haine 2008) as well as anemones hosting the symbiotic alpheid shrimp Alpheus armatus maintenance behaviors (Heil and McKey 2003; Stier et al. are significantly less likely to sustain damage by H. carunculata 2012) and defensive social interactions (Glynn 1980; Brooks than anemones without this shrimp. Our results suggest that the and Gwaltney 1993; Heil and McKey 2003;McKeonetal. association between A. armatus and B. annulata, although com- 2012). Previous studies have demonstrated that some crusta- plex because of the numerous symbionts involved, may be closer ceans will actively defend host cnidarians in their natural to mutualism on the symbiotic continuum. -

Download-The-Data (Accessed on 12 July 2021))
diversity Article Integrative Taxonomy of New Zealand Stenopodidea (Crustacea: Decapoda) with New Species and Records for the Region Kareen E. Schnabel 1,* , Qi Kou 2,3 and Peng Xu 4 1 Coasts and Oceans Centre, National Institute of Water & Atmospheric Research, Private Bag 14901 Kilbirnie, Wellington 6241, New Zealand 2 Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China; [email protected] 3 College of Marine Science, University of Chinese Academy of Sciences, Beijing 100049, China 4 Key Laboratory of Marine Ecosystem Dynamics, Second Institute of Oceanography, Ministry of Natural Resources, Hangzhou 310012, China; [email protected] * Correspondence: [email protected]; Tel.: +64-4-386-0862 Abstract: The New Zealand fauna of the crustacean infraorder Stenopodidea, the coral and sponge shrimps, is reviewed using both classical taxonomic and molecular tools. In addition to the three species so far recorded in the region, we report Spongicola goyi for the first time, and formally describe three new species of Spongicolidae. Following the morphological review and DNA sequencing of type specimens, we propose the synonymy of Spongiocaris yaldwyni with S. neocaledonensis and review a proposed broad Indo-West Pacific distribution range of Spongicoloides novaezelandiae. New records for the latter at nearly 54◦ South on the Macquarie Ridge provide the southernmost record for stenopodidean shrimp known to date. Citation: Schnabel, K.E.; Kou, Q.; Xu, Keywords: sponge shrimp; coral cleaner shrimp; taxonomy; cytochrome oxidase 1; 16S ribosomal P. Integrative Taxonomy of New RNA; association; southwest Pacific Ocean Zealand Stenopodidea (Crustacea: Decapoda) with New Species and Records for the Region.