Rev 10/6/2015, 1 PROJECT PROTOCOL Quality in Anti-Incontinence Surgery
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Urology Services in the ASC
Urology Services in the ASC Brad D. Lerner, MD, FACS, CASC Medical Director Summit ASC President of Chesapeake Urology Associates Chief of Urology Union Memorial Hospital Urologic Consultant NFL Baltimore Ravens Learning Objectives: Describe the numerous basic and advanced urology cases/lines of service that can be provided in an ASC setting Discuss various opportunities regarding clinical, operational and financial aspects of urology lines of service in an ASC setting Why Offer Urology Services in Your ASC? Majority of urologic surgical services are already outpatient Many urologic procedures are high volume, short duration and low cost Increasing emphasis on movement of site of service for surgical cases from hospitals and insurance carriers to ASCs There are still some case types where patients are traditionally admitted or placed in extended recovery status that can be converted to strictly outpatient status and would be suitable for an ASC Potential core of fee-for-service case types (microsurgery, aesthetics, prosthetics, etc.) Increasing Population of Those Aged 65 and Over As of 2018, it was estimated that there were 51 million persons aged 65 and over (15.63% of total population) By 2030, it is expected that there will be 72.1 million persons aged 65 and over National ASC Statistics - 2017 Urology cases represented 6% of total case mix for ASCs Urology cases were 4th in median net revenue per case (approximately $2,400) – behind Orthopedics, ENT and Podiatry Urology comprised 3% of single specialty ASCs (5th behind -

EAU Guidelines on Urinary Incontinence in Adults
EAU Guidelines on Urinary Incontinence in Adults F.C . Burkhard (Chair), J.L.H.R. Bosch, F. Cruz, G.E. Lemack, A.K. Nambiar, N. Thiruchelvam, A. Tubaro Guidelines Associates: D. Ambühl, D.A. Bedretdinova, F. Farag, R. Lombardo, M.P. Schneider © European Association of Urology 2018 TABLE OF CONTENTS PAGE 1. INTRODUCTION 8 1.1 Aim and objectives 8 1.1.1 The elderly 8 1.2 Panel composition 8 1.3 Available publications 8 1.4 Publication history 9 1.4.1 Summary of changes. 9 2. METHODS 11 2.1 Introduction 11 2.2 Review 11 2.3 Future goals 11 3. DIAGNOSTIC EVALUATION 11 3.1 History and physical examination 11 3.2 Patient questionnaires 12 3.2.1 Questions 12 3.2.2 Evidence 12 3.2.3 Summary of evidence and recommendations for patient questionnaires 13 3.3 Voiding diaries 14 3.3.1 Question 14 3.3.2 Evidence 14 3.3.3 Summary of evidence and recommendations for voiding diaries 14 3.4 Urinalysis and urinary tract infection 14 3.4.1 Question 14 3.4.2 Evidence 14 3.4.3 Summary of evidence and recommendations for urinalysis 15 3.5 Post-void residual volume 15 3.5.1 Question 15 3.5.2 Evidence 15 3.5.3 Summary of evidence and recommendations for post-void residual 15 3.6 Urodynamics 15 3.6.1 Question 16 3.6.2 Evidence 16 3.6.2.1 Variability 16 3.6.2.2 Diagnostic accuracy 16 3.6.2.3 Question 16 3.6.2.4 Evidence 16 3.6.2.5 Question 16 3.6.2.6 Evidence 16 3.6.2.7 Question 17 3.6.2.8 Evidence 17 3.6.2.9 Question 17 3.6.2.10 Evidence 17 3.6.3 Summary of evidence and recommendations for urodynamics 17 3.6.4 Research priority 18 3.7 Pad testing 18 3.7.1 Questions 18 3.7.2 Evidence 18 3.7.3 Summary of evidence and recommendations for pad testing 18 3.7.4 Research priority 18 3.8 Imaging 18 3.8.1 Questions 19 3.8.2 Evidence 19 3.8.3 Summary of evidence and recommendations for imaging 19 3.8.4 Research priority 19 2 URINARY INCONTINENCE IN ADULTS - LIMITED UPDATE MARCH 2018 4. -

Invasive Treatments for Urinary Incontinence
Cigna Medical Coverage Policy Effective Date .......................... 12/15/2013 Subject Invasive Treatments for Next Review Date .................... 12/15/2014 Coverage Policy Number ................. 0365 Urinary Incontinence Table of Contents Hyperlink to Related Coverage Policies Coverage Policy .................................................. 1 Biofeedback General Background ........................................... 2 Botulinum Therapy Coding/Billing Information ................................. 15 Electrical Stimulators References ........................................................ 16 Extracorporeal Electromagnetic Stimulation for Urinary Incontinence Injectable Bulking Agents for Urinary Conditions and Fecal Incontinence Physical Therapy Sacral Nerve Stimulation for Urinary Voiding Dysfunction and Fecal Incontinence INSTRUCTIONS FOR USE The following Coverage Policy applies to health benefit plans administered by Cigna companies. Coverage Policies are intended to provide guidance in interpreting certain standard Cigna benefit plans. Please note, the terms of a customer’s particular benefit plan document [Group Service Agreement, Evidence of Coverage, Certificate of Coverage, Summary Plan Description (SPD) or similar plan document] may differ significantly from the standard benefit plans upon which these Coverage Policies are based. For example, a customer’s benefit plan document may contain a specific exclusion related to a topic addressed in a Coverage Policy. In the event of a conflict, a customer’s benefit plan document -

Coders' Desk Reference for ICD-10-PCS Procedures
2 0 2 DESK REFERENCE 1 ICD-10-PCS Procedures ICD-10-PCS for DeskCoders’ Reference Coders’ Desk Reference for ICD-10-PCS Procedures Clinical descriptions with answers to your toughest ICD-10-PCS coding questions Sample 2021 optum360coding.com Contents Illustrations ..................................................................................................................................... xi Introduction .....................................................................................................................................1 ICD-10-PCS Overview ...........................................................................................................................................................1 How to Use Coders’ Desk Reference for ICD-10-PCS Procedures ...................................................................................2 Format ......................................................................................................................................................................................3 ICD-10-PCS Official Guidelines for Coding and Reporting 2020 .........................................................7 Conventions ...........................................................................................................................................................................7 Medical and Surgical Section Guidelines (section 0) ....................................................................................................8 Obstetric Section Guidelines (section -

Vaginal Reconstruction/Sling Urethropexy)
Patient Name: _ Date: _ New Jersey Urologic Institute Dr Betsy Greenleaf DO, FACOOG Pelvic Medicine and Reconstructive Surgery 10Industrial Way East, Suite 101, Eatontown, New Jersey 07724 732-963-9091 Fax: 732-963-9092 Findings: _ Post Operative Instructions (Vaginal Reconstruction/Sling Urethropexy) 1. Activity: May do as much as you feel up to. Your body will let you know when you are doing too much. Don't push yourself, however. Walking is ok and encouraged. If you sit too long you will become stiff and it will make it more difficult to move. Lying around can promote the formation of blood clots that can be life threatening. It is therefore important to move around. If you don't feel like walking, at least move your legs around in bed from time to time. Stairs are ok, just be careful of standing up too quickly and becoming light headed. Sitting still can also increase your risk of pneumonia. In addition to moving around, practice taking deep breaths ( 10 times each every hour or so) to keep your lungs properly aerated. Limitations: Avoid lifting or pushing/pulling any objects heavier than 1Olbs for at least 3 months. For patients with pelvic hernia or prolapse repairs it is recommended not to lift objects heavier than 25 Ibs for life. This may seem unrealistic. Try to put off lifting as long as possible. If you must lift, do not hold your breathe. Blow out as you lift to decrease abdominal and pelvic pressure. Also be aware that if you choose to lift objects heavier than recommended you risk forming another hernia 2. -

Video Surgi Session 2 11:00Am - 1:00Pm Thursday, 29Th October, 2020
Video Surgi Session 2 11:00am - 1:00pm Thursday, 29th October, 2020 41 Holmium Laser Ureterocele Excision with Transurethral Incision of the Prostate Grant R. Pollock MD1, Kalpesh Patel MD2, Joel Funk MD1 1University of Arizona, Department of Urology, Tucson, AZ, USA. 2Arizona Institute of Urology, Tucson, AZ, USA Abstract Objectives: Ureteroceles present a diagnostic and treatment challenge in adults. With an estimated prevalence of 1/500 to 1/4000, it is not uncommon for any urologist to encounter a ureterocele in clinical practice. We present an interesting case of a 53-year-old male with a 20-year history of obstructive voiding symptoms who presented to clinic with urinary retention that was found to be secondary to an orthotopic ureterocele that was prolapsed into the prostatic urethra. The patient underwent holmium laser ureterocele excision with transurethral incision of the prostate with a successful outcome. We present a video demonstrating the technique. Materials and Methods: Preoperative evaluation included a transrectal ultrasound of the prostate which revealed a prostate volume of 20cc. Urodynamics was also performed and pressure flow studies revealed a high-pressure, low flow voiding pattern with a functional detrusor muscle. Cystourethroscopy was performed revealing that an orthotopic ureterocele on the left side was prolapsed into the prostatic urethra and the bladder neck was mildly elevated. Using MOSES technology and laser settings of 30 Hz and 1.5 J, the ureterocele was completely excised and a transurethral incision of the prostate was performed. Results: The patient was discharged home on the day of surgery in stable condition with a Foley catheter in place. -
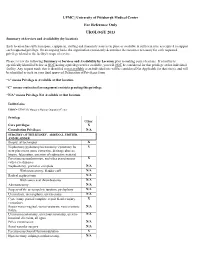
Summary of Services and Availability (By Location)
UPMC | University of Pittsburgh Medical Center For Reference Only UROLOGY 2013 Summary of Services and Availability (by location) Each location has sufficient space, equipment, staffing and financial resources in place or available in sufficient time as required to support each requested privilege. On an ongoing basis, the organization consistently determines the resources necessary for each requested privilege related to the facility's scope of service. Please review the following Summary of Services and Availability by Location prior to making your selections. If a facility is specifically identified below as NOT having a privilege/service available, you will NOT be considered for that privilege at that individual facility. Any request made that is identified as not available at an individual site will be considered Not Applicable for that site(s), and will be identified as such on your final approved Delineation of Privileges form. “x” means Privilege is Available at that location. “C” means contractual arrangement restricts granting this privilege. “N/A” means Privilege Not Available at that location. Facility Codes: UHOC= UPMC St. Margaret Harmar Outpatient Center Privilege UHOC Core privileges X Consultation Privileges N/A SURGERY OF THE KIDNEY, ADRENAL, URETER, AND BLADDER Biopsy, all techniques X Nephrotomy/pyelotomy/ureterotomy/ cystotomy for X stent placement, stone extraction, drainage abscess, biopsy, fulgeration, insertion of radioactive material Percutaneous nephroscopy, and other percutaneous X catheter techniques Nephrectomy, -
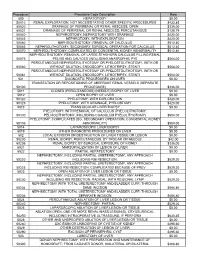
Procedure Procedure Code Description Rate 500
Procedure Procedure Code Description Rate 500 HEPATOTOMY $0.00 50010 RENAL EXPLORATION, NOT NECESSITATING OTHER SPECIFIC PROCEDURES $433.85 50020 DRAINAGE OF PERIRENAL OR RENAL ABSCESS; OPEN $336.00 50021 DRAINAGE OF PERIRENAL OR RENAL ABSCESS; PERCUTANIOUS $128.79 50040 NEPHROSTOMY, NEPHROTOMY WITH DRAINAGE $420.00 50045 NEPHROTOMY, WITH EXPLORATION $420.00 50060 NEPHROLITHOTOMY; REMOVAL OF CALCULUS $512.40 50065 NEPHROLITHOTOMY; SECONDARY SURGICAL OPERATION FOR CALCULUS $512.40 50070 NEPHROLITHOTOMY; COMPLICATED BY CONGENITAL KIDNEY ABNORMALITY $512.40 NEPHROLITHOTOMY; REMOVAL OF LARGE STAGHORN CALCULUS FILLING RENAL 50075 PELVIS AND CALYCES (INCLUDING ANATROPHIC PYE $504.00 PERCUTANEOUS NEPHROSTOLITHOTOMY OR PYELOSTOLITHOTOMY, WITH OR 50080 WITHOUT DILATION, ENDOSCOPY, LITHOTRIPSY, STENTI $504.00 PERCUTANEOUS NEPHROSTOLITHOTOMY OR PYELOSTOLITHOTOMY, WITH OR 50081 WITHOUT DILATION, ENDOSCOPY, LITHOTRIPSY, STENTI $504.00 501 DIAGNOSTIC PROCEDURES ON LIVER $0.00 TRANSECTION OR REPOSITIONING OF ABERRANT RENAL VESSELS (SEPARATE 50100 PROCEDURE) $336.00 5011 CLOSED (PERCUTANEOUS) (NEEDLE) BIOPSY OF LIVER $0.00 5012 OPEN BIOPSY OF LIVER $0.00 50120 PYELOTOMY; WITH EXPLORATION $420.00 50125 PYELOTOMY; WITH DRAINAGE, PYELOSTOMY $420.00 5013 TRANSJUGULAR LIVER BIOPSY $0.00 PYELOTOMY; WITH REMOVAL OF CALCULUS (PYELOLITHOTOMY, 50130 PELVIOLITHOTOMY, INCLUDING COAGULUM PYELOLITHOTOMY) $504.00 PYELOTOMY; COMPLICATED (EG, SECONDARY OPERATION, CONGENITAL KIDNEY 50135 ABNORMALITY) $504.00 5014 LAPAROSCOPIC LIVER BIOPSY $0.00 5019 OTHER DIAGNOSTIC PROCEDURES -

Burch Colposuspension
Burch Colposuspension Ericka M. Sohlberg, MDa, Christopher S. Elliott, MD, PhDa,b,* KEYWORDS Burch Colposuspension Urethropexy KEY POINTS Stress urinary incontinence is a prevalent condition for which surgical treatment continues to evolve. The Burch colposuspension has a 50-plus year history demonstrating strong long-term outcomes with few complications. Laparoscopic and open Burch colposuspension approaches have been shown to have equal efficacy. Other minimally invasive options, such as the mini-incisional Burch and robotic Burch, have less comparison data, although likely have similar outcomes. Although the use of the Burch colposuspension has waned in recent years secondary to a shift to- ward urethral sling operations, the Burch procedure still has an important role in the treatment of stress incontinence; specifically, a Burch should be considered when vaginal access is limited, intra-abdominal concurrent surgery is planned, or mesh is contraindicated. INTRODUCTION In fact, over the past 2 decades, minimally invasive urethral sling procedures have become the domi- Stress urinary incontinence (SUI) is a prevalent nant form of SUI treatment in the United States, ac- condition affecting 25% to 35% of the US female 1–3 counting for nearly 90% of all surgical corrections population. The current lifetime risk of surgery in 2009.7,8 However, following the 2011 Food and for SUI in the United States is approximately Drug Administration notification on serious compli- 13.5% with an estimated 200,000 women under- 4,5 cations associated with transvaginal mesh, the going surgical repair annually. These rates are negative publicity associated with vaginal synthetic predicted to increase in the coming years second- 9,10 6 mesh products has extended to urethral slings. -

Surgery for Urinary Incontinence in Women
Committee 14 Surgery for Urinary Incontinence in Women Chair A.R.B. SMITH (U.K) Members R. DMOCHOWSKI (USA), P. H ILTON (U.K), E. ROVNER (USA), C.G. NILSSON (Fin) Consultants F.M. REID (U.K), D. CHANG (USA) 1191 CONTENTS INTRODUCTION VI. COMPLICATIONS OF SURGERY FOR DETRUSOR OVERACTIVITY I. SURGERY FOR STRESS INCONTINENCE VII. NEUROMODULATION VIII. URETHRAL DIVERTICULUM II. CONFOUNDING VARIABLES IX. NON-OBSTETRIC URINARY III. STRESS INCONTINENCE WITH FISTULAE PROLAPSE X. OUTCOME MEASURES IV. COMPLICATIONS OF SURGERY FOR STRESS INCONTINENCE IX. ARTIFICIAL URINARY SPHINCTER IN WOMEN V. SURGERY FOR DETRUSOR OVERACTIVITY REFERENCES 1192 Surgery for Urinary Incontinence in Women A.R.B. SMITH R. DMOCHOWSKI, P. HILTON, E. ROVNER, C.G. NILSSON, F.M. REID, D. CHANG literature available at the time (Black & Downs, 1996, INTRODUCTION Downs & Black, 1996) [2,3]. In particular they highlighted the difficulties posed by case definition Surgeons have been criticised for the lack of rigour and the uniformity of surgical technique both between with which they have analysed their results. This and within surgical teams, the problem of variations chapter highlights the progress made, particularly in in outcome measures used, the handling of con- surgery for stress incontinence where more founding variables, the generalisability or external randomised controlled trials have been reported, and validity of the results, and the lack of statistical power illustrates areas where more studies are needed. The in most studies. Most systematic reviews published section on outcome measures illustrates that whilst since on the topic of surgery for incontinence in general the measurement of subjective and objective outcome or relating to individual surgical procedures have may clarify the value of an operation some standa- made similar comments regarding the quality of rdisation of outcome measures is required in order to available evidence. -

Medical Policy Invasive Treatment for Urinary Incontinence
Medical Policy Invasive Treatment for Urinary Incontinence Subject: Invasive Treatment for Urinary Incontinence Background: Urinary incontinence (the involuntary loss of urine) is a symptom that can be caused by a wide range of conditions including bladder dysfunction, sphincter incompetence, prostate problems (e.g., benign prostatic hypertrophy, prostatic carcinoma) or nerve damage. Symptoms of urinary incontinence (UI) can range from mild leaking to uncontrollable wetting. UI typically becomes more common with age, and women experience symptoms more often than men. There are four prevalent types of UI that occur in adults: • Stress Incontinence: The most common type of leakage, stress incontinence typically occurs during physical movement or activity (e.g., coughing, sneezing, running, heavy lifting) that puts pressure on the bladder. The primary causes of stress incontinence are urethral sphincter weakness (intrinsic sphincter deficiency) or a hypermobile urethra that occurs when there is weakness of pelvic floor and poor support of the vesicourethral sphincter unit. • Urge incontinence: Often referred to as "overactive bladder", urge incontinence is the unintentional loss of urine caused by the contraction of an overactive detrusor muscle (smooth muscle found in wall of bladder), usually associated with a sense of urgency. Urge incontinence is more commonly seen in men. • Overflow incontinence: Characterized by frequent small urinations and dribbling, overflow incontinence occurs when the bladder is full and unable to empty. Overflow incontinence is most common in men with a history of surgery or prostate problems, and rare in women. • Mixed incontinence: Mixed incontinence most commonly refers to a combination of stress and urge incontinence. Diagnostic evaluation for UI includes a complete history and physical, urinalysis, and diagnostic testing including urodynamic testing (if indicated) to assess urinary tract function, bladder filling and storage, and bladder emptying. -

Section 13 Urinary System
SECTION 13 URINARY SYSTEM The urinary system has several important functions, including: (1) constantly filtering blood to remove waste materials and urea (2) maintaining proper balance of water, salts, and acids (3) removing excess fluids from the body Major organs found in this system are: two kidneys two ureters (tubes from the kidneys to the bladder) one urinary bladder (both ureters drain into this bladder) one urethra (tube from bladder to outside) Word Elements (We will first look at some of the word elements that might be used in this system. Listen as each word element is being pronounced. Practice these word elements several times before going on to the next section.) -algia (al’ je a) means pain or suffering algia -cele means tumor or swelling, hernia, cyst cele cortic/o (kor ti ko) means outer region or cortex cortico cyst/o (sis to) means sac of fluid or bladder cysto dia- (di a) means through or complete dia dys- (dis) means bad, labored, difficult, painful dys -ectasis (ek’ ta sis) means stretching, dilation, enlargement ectasis -ectomy (ek’ to me) means surgical removal, called excision ectomy -emia (em e a) means blood condition emia epi- (ep i) means above or over epi glomerul/o (glo mer’ u lo) means ball or cluster, as in glomerulus glomerulo -gram (gram) means record or image gram -graphy (graf e) means the procedure of recording or writing graphy hem/o (he mo) means blood hemo hydr/o (hi dro) means water or fluid hydro hyp/o (hi po) means below or beneath hypo inter- (in ter) means between inter intra- (in tra) means