MAMMALIAN SPECIES NO. 222, Pp. I-A, 4 Ñgs
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
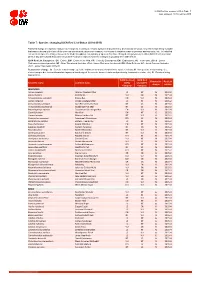
Table 7: Species Changing IUCN Red List Status (2014-2015)
IUCN Red List version 2015.4: Table 7 Last Updated: 19 November 2015 Table 7: Species changing IUCN Red List Status (2014-2015) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2014 (IUCN Red List version 2014.3) and 2015 (IUCN Red List version 2015-4) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2014) List (2015) change version Category Category MAMMALS Aonyx capensis African Clawless Otter LC NT N 2015-2 Ailurus fulgens Red Panda VU EN N 2015-4 -

Dental and Temporomandibular Joint Pathology of the Kit Fox (Vulpes Macrotis)
Author's Personal Copy J. Comp. Path. 2019, Vol. 167, 60e72 Available online at www.sciencedirect.com ScienceDirect www.elsevier.com/locate/jcpa DISEASE IN WILDLIFE OR EXOTIC SPECIES Dental and Temporomandibular Joint Pathology of the Kit Fox (Vulpes macrotis) N. Yanagisawa*, R. E. Wilson*, P. H. Kass† and F. J. M. Verstraete* *Department of Surgical and Radiological Sciences and † Department of Population Health and Reproduction, School of Veterinary Medicine, University of California, Davis, California, USA Summary Skull specimens from 836 kit foxes (Vulpes macrotis) were examined macroscopically according to predefined criteria; 559 specimens were included in this study. The study group consisted of 248 (44.4%) females, 267 (47.8%) males and 44 (7.9%) specimens of unknown sex; 128 (22.9%) skulls were from young adults and 431 (77.1%) were from adults. Of the 23,478 possible teeth, 21,883 teeth (93.2%) were present for examina- tion, 45 (1.9%) were absent congenitally, 405 (1.7%) were acquired losses and 1,145 (4.9%) were missing ar- tefactually. No persistent deciduous teeth were observed. Eight (0.04%) supernumerary teeth were found in seven (1.3%) specimens and 13 (0.06%) teeth from 12 (2.1%) specimens were malformed. Root number vari- ation was present in 20.3% (403/1,984) of the present maxillary and mandibular first premolar teeth. Eleven (2.0%) foxes had lesions consistent with enamel hypoplasia and 77 (13.8%) had fenestrations in the maxillary alveolar bone. Periodontitis and attrition/abrasion affected the majority of foxes (71.6% and 90.5%, respec- tively). -

An Educator's Resource to Texas Mammal Skulls and Skins
E4H-014 11/17 An Educator’s Resource to Texas Mammal Skulls and Skins for use in 4-H Wildlife Programs and FFA Wildlife Career Development Events By, Denise Harmel-Garza Program Coordinator I, Texas A&M AgriLife Extension Service, 4-H Photographer and coauthor, Audrey Sepulveda M.Ed. Agricultural Leadership, Education and Communications, Texas A&M University College Station, Texas 2017 “A special thanks to the Biodiversity Research and Teaching Collections at Texas A&M University for providing access to their specimens.” Texas A&M AgriLife Extension provides equal opportunities in its programs and employment to all persons, regardless of race, color, sex, religion, national origin, disability, age, genetic information, veteran status, sexual orientation, or gender identity. The Texas A&M University System, U.S. Department of Agriculture, and the County Commissioners Courts of Texas Cooperating. Introduction Texas youth that participate in wildlife programs may be asked to identify a skull, skin, scat, tracks, etc. of an animal. Usually, educators must find this information and assemble pictures of skulls and skins from various sources. They also must ensure that what they find is relevant and accurate. Buying skulls and skins to represent all Texas mammals is costly. Most educators cannot afford them, and if they can, maintaining these collections over time is problematic. This study resource will reduce the time teachers across the state need to spend searching for information and allow them more time for presenting the material to their students. This identification guide gives teachers and students easy access to information that is accurate and valuable for learning to identify Texas mammals. -

University of Florida Thesis Or Dissertation Formatting
UNDERSTANDING CARNIVORAN ECOMORPHOLOGY THROUGH DEEP TIME, WITH A CASE STUDY DURING THE CAT-GAP OF FLORIDA By SHARON ELIZABETH HOLTE A DISSERTATION PRESENTED TO THE GRADUATE SCHOOL OF THE UNIVERSITY OF FLORIDA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY UNIVERSITY OF FLORIDA 2018 © 2018 Sharon Elizabeth Holte To Dr. Larry, thank you ACKNOWLEDGMENTS I would like to thank my family for encouraging me to pursue my interests. They have always believed in me and never doubted that I would reach my goals. I am eternally grateful to my mentors, Dr. Jim Mead and the late Dr. Larry Agenbroad, who have shaped me as a paleontologist and have provided me to the strength and knowledge to continue to grow as a scientist. I would like to thank my colleagues from the Florida Museum of Natural History who provided insight and open discussion on my research. In particular, I would like to thank Dr. Aldo Rincon for his help in researching procyonids. I am so grateful to Dr. Anne-Claire Fabre; without her understanding of R and knowledge of 3D morphometrics this project would have been an immense struggle. I would also to thank Rachel Short for the late-night work sessions and discussions. I am extremely grateful to my advisor Dr. David Steadman for his comments, feedback, and guidance through my time here at the University of Florida. I also thank my committee, Dr. Bruce MacFadden, Dr. Jon Bloch, Dr. Elizabeth Screaton, for their feedback and encouragement. I am grateful to the geosciences department at East Tennessee State University, the American Museum of Natural History, and the Museum of Comparative Zoology at Harvard for the loans of specimens. -

PHYLOGENETIC SYSTEMATICS of the BOROPHAGINAE (CARNIVORA: CANIDAE) Xiaoming Wang
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Mammalogy Papers: University of Nebraska State Museum, University of Nebraska State Museum 1999 PHYLOGENETIC SYSTEMATICS OF THE BOROPHAGINAE (CARNIVORA: CANIDAE) Xiaoming Wang Richard H. Tedford Beryl E. Taylor Follow this and additional works at: http://digitalcommons.unl.edu/museummammalogy This Article is brought to you for free and open access by the Museum, University of Nebraska State at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Mammalogy Papers: University of Nebraska State Museum by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. PHYLOGENETIC SYSTEMATICS OF THE BOROPHAGINAE (CARNIVORA: CANIDAE) XIAOMING WANG Research Associate, Division of Paleontology American Museum of Natural History and Department of Biology, Long Island University, C. W. Post Campus, 720 Northern Blvd., Brookville, New York 11548 -1300 RICHARD H. TEDFORD Curator, Division of Paleontology American Museum of Natural History BERYL E. TAYLOR Curator Emeritus, Division of Paleontology American Museum of Natural History BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY Number 243, 391 pages, 147 figures, 2 tables, 3 appendices Issued November 17, 1999 Price: $32.00 a copy Copyright O American Museum of Natural History 1999 ISSN 0003-0090 AMNH BULLETIN Monday Oct 04 02:19 PM 1999 amnb 99111 Mp 2 Allen Press • DTPro System File # 01acc (large individual, composite figure, based Epicyon haydeni ´n. (small individual, based on AMNH 8305) and Epicyon saevus Reconstruction of on specimens from JackNorth Swayze America. Quarry). Illustration These by two Mauricio species Anto co-occur extensively during the late Clarendonian and early Hemphillian of western 2 1999 WANG ET AL.: SYSTEMATICS OF BOROPHAGINAE 3 CONTENTS Abstract .................................................................... -

H420/02 Biological Diversity
A Level Biology A H420/02 Biological Diversity Question Set 11 1 The cheetah, Acinonyx jubatus, is a member of the cat family, Felidae. Cheetahs display less intraspecific variation than other members of the family Felidae. Fig. 20.1 shows the mean body length of a population of cheetahs from southern Africa. The error bars on Fig. 20.1 show the standard deviation of mean body length. Fig. 20.1 (a) (i) At between 2.5 and 4 years old, the mean length of female cheetahs is less than that of males. Calculate how much shorter than males female cheetahs are. Show your working. Express your answer as a percentage to two significant figures. Answer……………% [2] (ii) Using only Fig. 20.1 and your answer to (i), what can be concluded about the significance of the difference between the length of male and female cheetahs aged between 2.5 and 4 years? Explain your answer. [2] (iii) A student looked at Fig. 20.1 and wrote: “The longest male cheetah that was measured was 1.52 m long”. Explain whether the information in Fig. 20.1 supports the student’s answer. [1] (iv) State the likely causes of variation in body length in cheetahs. [2] (b) The population of cheetahs has been declining for the past 100 years and is estimated to be between 6000 and 7000. Within the remaining cheetah population, intraspecific genetic diversity is very low. One isolated population of cheetahs in Iran has fewer than 100 individuals. (i) State one way in which genetic diversity can be measured. -

Comparative Craniometric Measurements of Two Sympatric Species of Vulpes in Ikh Nart Nature Reserve, Mongolia
© 2018 Journal compilation ISSN 1684-3908 (print edition) http://mjbs.num.edu.mn Mongolian Journal of Biological http://biotaxa.org./mjbs Sciences MJBS Volume 16(1), 2018 ISSN 2225-4994 (online edition) http://dx.doi.org/10.22353/mjbs.2018.16.03 Original Article Comparative Craniometric Measurements of Two Sympatric Species of Vulpes in Ikh Nart Nature Reserve, Mongolia Tserendorj Munkhzul1, Richard P. Reading2, Bayarbaatar Buuveibaatar3 & James D. Murdoch4 1Mammalian Ecology Laboratory, Institute of General and Experimental Biology, Mongolian Academy of Sciences, Ulaanbaatar, Mongolia 2International Conservation Coalition, Denver, Colorado 80220 USA, Butterfly Pavilion, Westminster, Colorado 80020 USA & Mongolian Conservation Coalition, Ulaanbaatar, Mongolia 3Wildlife Conservation Society, Mongolia Program, Ulaanbaatar, Mongolia 4Rubenstein School of Environment and Natural Resources, University of Vermont, George Aiken Center, Burlington, Vermont 05405 USA Abstract Key words: Corsac fox; In Mongolia, both the red fox (Vulpes vulpes) and corsac fox (Vulpes corsac) occupy cranium; morphometry; broad sympatric ranges, but despite their expansive ranges, few published details of red fox; Vulpes; skull. the craniometry of either species exist in Mongolia and other parts of northern and Article information: central Asia. To determine the morphological differences between two species of Received: 08 Febr. 2018 foxes, we tested for morphological and morphometrical differences between the red Accepted: 31 May 2018 (n = 13) and corsac (n = 11) foxes using 63 cranium measurements. All significantly Published online: different skull variables were larger for red foxes than corsac foxes. This paper 12 June 2018 reports comparison of the cranial measurements from skulls of red and corsac foxes Correspondence: and serves as a preliminary investigation of interspecific variation between these [email protected] species. -

Return of a Lost Structure in the Evolution of Felid Dentition Revisited: a Devoevo Perspective on the Irreversibility of Evolution
bioRxiv preprint doi: https://doi.org/10.1101/2021.02.04.429820; this version posted February 5, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. RUNNING HEAD: LYNX M2 AND IRREVERSIBILE EVOLUTION Return of a lost structure in the evolution of felid dentition revisited: A DevoEvo perspective on the irreversibility of evolution Vincent J. Lynch Department of Biological Sciences, University at Buffalo, SUNY, 551 Cooke Hall, Buffalo, NY, 14260, USA. Correspondence: [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/2021.02.04.429820; this version posted February 5, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. RUNNING HEAD: LYNX M2 AND IRREVERSIBILE EVOLUTION Abstract There is a longstanding interest in whether the loss of complex characters is reversible (so-called “Dollo’s law”). Reevolution has been suggested for numerous traits but among the first was Kurtén (1963), who proposed that the presence of the second lower molar (M2) of the Eurasian lynx (Lynx lynx) was a violation of Dollo’s law because all other Felids lack M2. While an early and often cited example for the reevolution of a complex trait, Kurtén (1963) and Werdelin (1987) used an ad hoc parsimony argument to support their proposition that M2 reevolved in Eurasian lynx. Here I revisit the evidence that M2 reevolved in Eurasian lynx using explicit parsimony and maximum likelihood models of character evolution and find strong evidence that Kurtén (1963) and Werdelin (1987) were correct – M2 reevolved in Eurasian lynx. -

Anatomy of the Red Panda (Ailurus Fulgens)
Journal of Biology and Life Science ISSN 2157-6076 2019, Vol. 10, No. 1 Anatomy of the Red Panda (Ailurus fulgens) Modesta Makungu (Corresponding Author) Department of Veterinary Surgery and Theriogenology, College of Veterinary Medicine and Biomedical Sciences, Sokoine University of Agriculture, P O Box 3020, Morogoro, Tanzania. Email: [email protected]; [email protected] Received: September 21, 2018 Accepted: October 19, 2018 doi:10.5296/jbls.v10i1.13677 URL: https://doi.org/10.5296/jbls.v10i1.13677 Abstract The red panda (Ailurus fulgens) is an endangered species primarily distributed in the southern China and Himalayas. It lives in mountain forests with bamboo understory. This review outlines the normal anatomy of the red panda in terms of its musculoskeletal system, respiratory system, circulatory system, digestive system and urogenital system. Knowledge of the normal anatomy of individual animal species is important for species identification and accurate interpretation and diagnosis of diseases. Keywords: anatomy, red panda 1. Introduction The red panda (Ailurus fulgens) (Figure 1) is classified as an endangered species by the International Union for Conservation of Nature and Natural Resources (IUCN, 2018). It belongs to order; Carnivora, family; Ailuridae, and genus; Ailurus (Wei and Zhang, 2009; IUCN, 2018). The red panda is the only living species of the family Ailuridae and genus Ailurus and is closely related to mustelids, procyonids and skunks (Wei and Zhang, 2009; Groves, 2011). 13 Journal of Biology and Life Science ISSN 2157-6076 2019, Vol. 10, No. 1 Figure 1. A photograph of an adult red panda (Ailurus fulgens fulgens) It is primarily distributed in India, Burma, China, Bhutan and Nepal (Roberts and Gittleman, 1984; Ghose and Dutta, 2011). -

Bayesian Phylogenetic Estimation of Fossil Ages Rstb.Royalsocietypublishing.Org Alexei J
Downloaded from http://rstb.royalsocietypublishing.org/ on June 20, 2016 Bayesian phylogenetic estimation of fossil ages rstb.royalsocietypublishing.org Alexei J. Drummond1,2 and Tanja Stadler2,3 1Department of Computer Science, University of Auckland, Auckland 1010, New Zealand 2Department of Biosystems Science and Engineering, Eidgeno¨ssische Technische Hochschule Zu¨rich, 4058 Basel, Switzerland Research 3Swiss Institute of Bioinformatics (SIB), Lausanne, Switzerland Cite this article: Drummond AJ, Stadler T. Recent advances have allowed for both morphological fossil evidence 2016 Bayesian phylogenetic estimation of and molecular sequences to be integrated into a single combined inference fossil ages. Phil. Trans. R. Soc. B 371: of divergence dates under the rule of Bayesian probability. In particular, 20150129. the fossilized birth–death tree prior and the Lewis-Mk model of discrete morphological evolution allow for the estimation of both divergence times http://dx.doi.org/10.1098/rstb.2015.0129 and phylogenetic relationships between fossil and extant taxa. We exploit this statistical framework to investigate the internal consistency of these Accepted: 3 May 2016 models by producing phylogenetic estimates of the age of each fossil in turn, within two rich and well-characterized datasets of fossil and extant species (penguins and canids). We find that the estimation accuracy of One contribution of 15 to a discussion meeting fossil ages is generally high with credible intervals seldom excluding the issue ‘Dating species divergences using rocks true age and median relative error in the two datasets of 5.7% and 13.2%, and clocks’. respectively. The median relative standard error (RSD) was 9.2% and 7.2%, respectively, suggesting good precision, although with some outliers. -

Genus/Species Skull Ht Lt Wt Stage Range Abacinonyx See Acinonyx Abathomodon See Speothos A
Genus/Species Skull Ht Lt Wt Stage Range Abacinonyx see Acinonyx Abathomodon see Speothos A. fossilis see Icticyon pacivorus? Pleistocene Brazil Abelia U.Miocene Europe Absonodaphoenus see Pseudarctos L.Miocene USA A. bathygenus see Cynelos caroniavorus Acarictis L.Eocene W USA cf. A. ryani Wasatchian Colorado(US) A. ryani Wasatchian Wyoming, Colorado(US) Acinomyx see Acinonyx Acinonyx M.Pliocene-Recent Europe,Asia,Africa,N America A. aicha 2.3 m U.Pliocene Morocco A. brachygnathus Pliocene India A. expectata see Miracinonyx expectatus? Or Felis expectata? A. intermedius M.Pleistocene A. jubatus living Cheetah M.Pliocene-Recent Algeria,Europe,India,China A. pardinensis 91 cm 3 m 60 kg Astian-Biharian Italy,India,China,Germany,France A. sp. L.Pleistocene Tanzania,Ethiopia A. sp. Cf. Inexpectatus Blancan-Irvingtonian California(US) A. studeri see Miracinonyx studeri Blancan Texas(US) A. trumani see Miracinonyx trumani Rancholabrean Wyoming,Nevada(US) Acionyx possibly Acinonyx? A. cf. Crassidens Hadar(Ethiopia) Acrophoca 1.5 m U.Miocene-L.Pliocene Peru,Chile A. longirostris U.Miocene-L.Pliocene Peru A. sp. U.Miocene-L.Pliocene Chile Actiocyon M-U.Miocene W USA A. leardi Clarendonian California(US) A. sp. M.Miocene Oregon(US) Adcrocuta 82 cm 1.5 m U.Miocene Europe,Asia A. advena A. eximia 80 cm 1.5 m Vallesian-Turolian Europe(widespread),Asia(widespread) Adelphailurus U.Miocene-L.Pliocene W USA, Mexico,Europe A. kansensis Hemphillian Arizona,Kansas(US),Chihuahua(Mexico) Adelpharctos M.Oligocene Europe Adilophontes L.Miocene W USA A. brachykolos Arikareean Wyoming(US) Adracodon probably Adracon Eocene France A. quercyi probably Adracon quercyi Eocene France Adracon U.Eocene-L.Oligocene France A. -

1: Beast2.1.3 R1
1: Beast2.1.3_r1 0.93 Canis adustus 0.89 Canis mesomelas 0.37 Canis simensis Canis arnensis 1 Lycaon pictus 1 Cuon javanicus 0.57 Xenocyon texanus 0.91 Canis antonii 0.96 0.35 Canis falconeri 1 Canis dirus 1 Canis armbrusteri 0.060.27 Canis lupus Canis chihliensis Canis etruscus 0.98 0.99 0.26 Canis variabilis 0.11 Canis edwardii 0.93 Canis mosbachensis 0.68 0.58 Canis latrans Canis aureus 0.94 Canis palmidens 0.94 Canis lepophagus 1 0.41 Canis thooides Canis ferox 0.99 Eucyon davisi 0.8 Cerdocyon texanus Vulpes stenognathus Leptocyon matthewi 0.98 Urocyon cinereoargenteus 0.85 Urocyon minicephalus 0.96 1 Urocyon citronus 1 Urocyon galushi 0.96 Urocyon webbi 1 1 Metalopex merriami 0.23 Metalopex macconnelli 0.68 Leptocyon vafer 1 Leptocyon leidyi Leptocyon vulpinus 0.7 Leptocyon gregorii 1 Leptocyon douglassi Leptocyon mollis 0.32 Borophagus dudleyi 1 Borophagus hilli 0.98 Borophagus diversidens 0.97 Borophagus secundus 0.18 Borophagus parvus 1 Borophagus pugnator 1 Borophagus orc 1 Borophagus littoralis 0.96 Epicyon haydeni 1 0.92 Epicyon saevus Epicyon aelurodontoides Protepicyon raki 0.98 0.28 Carpocyon limosus 0.96 Carpocyon webbi 0.97 0.98 Carpocyon robustus Carpocyon compressus 0.17 0.87 Paratomarctus euthos Paratomarctus temerarius Tomarctus brevirostris 0.31 0.85 0.35 Tomarctus hippophaga 0.17 Tephrocyon rurestris Protomarctus opatus 0.24 0.58 Microtomarctus conferta Metatomarctus canavus Psalidocyon marianae 0.79 1 Aelurodon taxoides 0.32 0.27 Aelurodon ferox 1 Aelurodon stirtoni 0.14 Aelurodon mcgrewi 0.33 Aelurodon asthenostylus