Oxycodone Import Policy Consultation Paper
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

In the United States District Court for the Eastern District of Pennsylvania ______: in Re Suboxone (Buprenorphine : Mdl No
IN THE UNITED STATES DISTRICT COURT FOR THE EASTERN DISTRICT OF PENNSYLVANIA __________________________________________ : IN RE SUBOXONE (BUPRENORPHINE : MDL NO. 2445 HYDROCHLORIDE AND NALOXONE) : 13-MD-2445 ANTITRUST LITIGATION : : THIS DOCUMENT RELATES TO:, : : Wisconsin, et al. v. Indivior Inc. et al. : Case No. 16-cv-5073 : __________________________________________: STATE OF WISCONSIN : By Attorney General Brad D. Schimel, et al. : : CIV. A. NO. 16-5073 Plaintiffs, : v. : : INDIVIOR INC. f/k/a RECKITT BENCKISER : PHARMACEUTICALS, INC., et al. : : Defendants. : __________________________________________: Goldberg, J. November 24, 2020 MEMORANDUM Defendant Reckitt Benckiser, Inc. (“Defendant”) manufactures Suboxone, a drug commonly used to combat opioid addiction.1 Suboxone previously came in tablet form, but in 2010, citing safety concerns, Defendant effectuated a change in the administration of this drug, switching from tablet to sublingual film. Various purchasers/consumers of Suboxone claimed that this switch was anticompetitive and solely designed to maintain Defendant’s market exclusivity—a scheme known as a “product hop.” These claims have resulted in multi-district, antitrust litigation before this Court. 1 Reckitt is currently known as Indivior, Inc. In December 2014, Reckitt Benckiser Pharmaceuticals, Inc. was demerged from its prior parent, the Reckitt Benckiser Group PLC, into Indivior PLC. Although Indivior is technically the named defendant in this case, the pleadings and many of the relevant exhibits use the name “Reckitt.” As discovery and class certification litigation have come to a close, the parties have raised numerous challenges under Daubert v. Merrell Dow Pharmaceuticals, 509 U.S. 579 (1993), seeking exclusion of all or selected portions of nine expert witnesses anticipated opinions. This Opinion explains my reasoning for the resolution of these motions and will hopefully set forth a clearer path towards trial. -

FREDERICK ('ERIC') RANDALL SMITH Bsc, Phd(Edin), FRSC Eric
FREDERICK ('ERIC') RANDALL SMITH BSc, PhD(Edin), FRSC Eric Smith was responsible for research activities in Duncan Flockhart Ltd, T & H Smith Ltd and Edinburgh Pharmaceutical Industries Ltd during the period 1947 to 1971. He and his colleagues made several lasting contributions to medicine and commerce notable amongst which were the creation of a new opiate industry based on large scale poppy growing in Australia, the establishment of dihydrocodeine ('DF118') as a major analgesic, and the discovery of 'Bitrex', an intensely bitter substance, now a widely used denaturant. He was elected a Fellow of the Society in 1962 and served on Council 1969-71. Born in Edinburgh on April 4th, 1911, Eric spent his childhood, his school and university days, and most of his working life and retirement in or near to the City. He died there on November 4th, 1994. Eric's enquiring attitude to life owed much to his father. This unusual man earned his living in the Actuarial Department of the Scottish Provident Institution but his main interests were astronomy and art. He was an FRAS and his personal observations led to his name being given to a minor lunar crater, and a good enough artist for his works to be hung in the Royal Academies in Edinburgh and London. Perhaps this is why Eric chose to be a scientist and his sister an art teacher. Eric was educated at the Edinburgh Institution (later named Melville College) where he was an excellent scholar and a good enough sportsman to play for the 1st XV and the FPs. -

Morphinone Reductase for the Preparation Of
Europäisches Patentamt *EP000649465B1* (19) European Patent Office Office européen des brevets (11) EP 0 649 465 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.7: C12N 9/02, C12P 17/18, of the grant of the patent: C12Q 1/32 24.07.2002 Bulletin 2002/30 (86) International application number: (21) Application number: 93913236.1 PCT/GB93/01129 (22) Date of filing: 28.05.1993 (87) International publication number: WO 94/00565 (06.01.1994 Gazette 1994/02) (54) MORPHINONE REDUCTASE FOR THE PREPARATION OF HYDROMORPHONE AND HYDROCODONE MORPHINONE REDUKTASE ZUR HERSTELLUNG VON HYDROMORPHONE UND HYDROCODONE MORPHINONE REDUCTASE DESTINEE A LA PREPARATION D’HYDROMORPHONE ET D’HYDROCODONE (84) Designated Contracting States: (74) Representative: Perry, Robert Edward AT BE CH DE DK ES FR GB IE IT LI NL PT SE GILL JENNINGS & EVERY Broadgate House (30) Priority: 25.06.1992 GB 9213524 7 Eldon Street London EC2M 7LH (GB) (43) Date of publication of application: 26.04.1995 Bulletin 1995/17 (56) References cited: WO-A-90/13634 (73) Proprietor: MACFARLAN SMITH LIMITED Edinburgh EH11 2QA (GB) • THE BIOCHEMICAL JOURNAL vol. 274, no. 3, 15 March 1991, pages 875 - 880 NEIL C. BRUCE ET (72) Inventors: AL. ’Microbial degradation of the morphine • HAILES, Anne, Maria 59 Lower Road alkaloids. Purification and characterization of Kent DA8 1AY (GB) morphine dehydrogenase from Pseudomonas • FRENCH, Christopher, Edward putida M10’ Palmerston North (NZ) • BRUCE, Neil, Charles Cambridge CB2 1ND (GB) Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. -

Does the International Narcotics Control Board
Clark JD, Johnson M, Fabowale B, Farrelly M, Currow D. Does the International Narcotics Control Board (INCB) sufficiently prioritise enablement of access to therapeutic opioids? A systematic critical analysis of six INCB annual reports, 1968-2018. Journal of Global Health Reports. 2020;4:e2020042. Research Articles Does the International Narcotics Control Board (INCB) sufficiently prioritise enablement of access to therapeutic opioids? A systematic critical analysis of six INCB annual reports, 1968-2018 Joseph D Clark 1, Miriam Johnson 1 , Blessing Fabowale 1, Michael Farrelly 2 , David Currow 3 1 Wolfson Palliative Care Research Centre, University of Hull, Hull, UK , 2 Faculty of Arts Cultures and Education, University of Hull, Hull, UK , 3 Wolfson Palliative Care Research Centre, University of Hull, Hull, UK; IMPACCT, Faculty of Health, University of Technology Sydney, Ultimo, Australia Keywords: opioids, analgesics, narcotics, drug legislation, controlled substances, pain management, palliative care Journal of Global Health Reports Vol. 4, 2020 Background The International Narcotics Control Board (INCB) has overseen international drug control since 1968 with the dual remit of restricting illicit production and use of controlled substances, whilst enabling access for clinical purposes. Two opioid crises are present under its jurisdiction: i) abuse, dependence and premature mortality in high-income countries; and ii) inadequate supply of opioids for clinical purposes for most of the world represented almost exclusively by low- and middle-income countries. Methods Systematic critical analysis using corpus linguistics as a method of document analysis to investigate the regulatory climate promoted by the INCB, through language used regarding opioids in a representative sample of annual Reports, 1968-2018. -

PHARMACEUTICALS + Biovian Oy Jemedic AB Bayer Oy + Shetland Bergen Helse Bergen HF, Haukeland 3
BARENTS SEA Jan Mayen (Norway) Tromsø NORWAY Murmansk DENMARK STRAIT NORWEGIAN SEA Raufarhöfn Bolungarvik Kalix Aromtech Oy Mosjøen SWEDEN Kemi Akureyri Luleå WHITE SEA Piteå Eskifjördur Oy Woikoski Ab + Pharmatory Ltd + Bioactive Bone Substitutes Oolu Fermion Oy Olafsvik ICELAND Skellefteå IA Hornafjördur Reykjavik 1-5 Grindavik Apotek Produktion & Laboratorier AB Oy Woikoski Ab Umeå Norrlands Universitetssjukhus Frøya Vik Trondheim Kristiansund GULF OF BOTHN Vaasa Averøya Galena Pharma Oy + Orion Oyj + Finvector Oy Sandøy Härnösand Ålesund Sundsvall Unimedic AB + MAP Medical Technologies Oy Woikoski Ab Kristinestad Tórshavn Apoteksverkiö, Framleiösluapotekiö Måløy EWOS AS Santen Oy Cytomed Oy SWEDEN - Not Shown Vitabalans Oy + Umeå + Curida AS Apotek Produktion & Laboratorier AB Oy Aga Ab Norrlands Universitetssjukhus Jemedic AB FINLAND OSLO Gävle Alby Vyborg + Nouryon Pulp & Performance Chemicals AB Hankintatukku Oy 1. Smerud Medical Research Norway AS Pcas Finland Oy Finex Oy Hamina Orion Corporation Oy Woikoski Ab 2. Catapult Life Science AS Gävle + + EUROPE - PHARMACEUTICALS + Biovian Oy Jemedic AB Bayer Oy + Shetland Bergen Helse Bergen HF, Haukeland 3. PhotoCure ASA Turku Orion Corporation A.Vogel Oy universitetssjukehus 4. GE Healthcare AS Vitabalans Oy MAP Medicals Technologies Oy + Nanoform Finland Oy Islands 5. Oncoinvent AS Fermion Oy AGA Gas AB Lumene Oy Lerwick 6. The Cyclotron Center Pharmaq AS + Scan Aqua AS Orion Corporation + 7. Oslo Universitetssykehus HF HELSINKI 8. Diatec Monoclonals AS Institute for Energy Technology -

United States Securities and Exchange Commission Form 10-K Shire Pharmaceuticals Group
UNITED STATES SECURITIES AND EXCHANGE COMMISSION WASHINGTON, D.C. 20549 FORM 10-K (Mark One) ፤ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 For the fiscal year ended December 31, 1999 អ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 Commission file number 0-29630 SHIRE PHARMACEUTICALS GROUP PLC (Exact name of registrant as specified in its charter) England and Wales (State or other jurisdiction (I.R.S. Employer of incorporation or organization) Identification No.) N.A. East Anton, Andover, Hampshire SP10 5RG England (Address of principal executive offices) (Zip Code) 44 1264 333455 (Registrant's telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: Title of each class Name of exchange on which registered American Depository Shares, each representing Nasdaq National Market 3 Ordinary Shares, 5 pence nominal value per share Securities registered pursuant to Section 12(g) of the Act: None (Title of class) Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ፤ No អ Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the Registrant's knowledge, in definitive proxy or information statements incorporated by reference to Part III of this Form 10-K or any amendment to this Form 10-K. -

August/September 2016
INTERNATIONAL PHARMACEUTICAL QUALITY IPQ Inside the Global Regulatory Dialogue IPQPUBS.COM VOL. 8, NO. 5 MONTHLY UPDATE - AUGUST/SEPTEMBER 2016 UNITED STATES ● Review/Inspection Control Strategy Communications Key to Advancing Biotech p. 4 Regulation, FDA Biotech Official Stresses ● More Clarity Sought in FDA’s Inactive Ingredient Database Regarding Drug Delivery Devices p. 13 EUROPE ● Protein Sequence Variants, Process Development and Spec Setting Are Generating Queries p. 20 from EU Biopharmaceutical CMC Assessors ● Procedures, Eligibilities and Goals of EMA’s Accelerated Access Efforts Are Taking More p. 32 Concrete Shape INTERNATIONAL ● Goal of FDA/EU Mutual Inspection Reliance is Nearing Realization p. 36 UPDATES IN BRIEF p. 47 U.S. CMC: ● GDUFA II Agreement ● ANDA Impurity Refusals ● Capsule Supplier Changes ● Co-Crystal Classification ● ANDA Impurity Refusals U.S. GMP: ● Insanitary Compounding ● NIH Clinical Manufacturing ● Establishment Registration ● Generics Facility ID'ing ● USP on Analytical Lifecycles EUROPE CMC: ● EMA Streamlining ● EMA Adaptive Pathways Workshop ● EDQM on ICH Q3D EUROPE GMP: ● EMA WFI Q&A ● EC on Plastics INTERNATIONAL CMC: ● Antimicrobial Resistance Roadmap ● WHO on Variations ● Canada on ICH Q3D INTERNATIONAL GMP: ● China on Drug Manufacturing ● India Manufacturing Self-Assessment FDA WARNING LETTERS, EMA NON-COMPLIANCE REPORTS, AND FDA RECALLS POSTED IN AUGUST/SEPTEMBER p. 51 MONTHLY UPDATE - AUGUST / SEPTEMBER 2016 INTERNATIONAL PHARMACEUTICAL QUALITY provides in-depth coverage of emerging drug, biologic and combination product CMC and GMP issues and developments with a mission of helping advance and harmonize the quality regulatory process globally. Headquartered in Washington, D.C., IPQ is read by regulatory agencies, manufacturers, suppliers, consultants, law firms, and universities around the world. -

United States Patent Office Patented Aug
3,830,819 United States Patent Office Patented Aug. 20, 1974 2 The invention provides a process for the purification of l-dihydrocodeine containing as impurities 1-dihydrothebai 3,830,819 MANUFACTURE OF l-DHYDROCODENE none and or 1-dihydrothebainol, which comprises treating Edward Leon Grew and David Jackson Powles, Edin the impure 1-dihydrocodeine to remove said impurities burgh, Scotland, assignors to MacFarlan Smith Limited, therefrom by either extraction with an aqueous alkali or Edinburgh, Scotland 5 by passage of the material through an anion exchange No Drawing. Fied Feb. 22, 1972, Ser. No. 228,367 resin of a quaternary ammonium type in the hydroxide Claims priority, application Great Britain, Feb. 22, 1971, form whereby the proportion of said impurities is reduced. 5,081/71 The crude 1-dihydrocodeine may be one prepared by Int, C. C07d 43/28 the catalytic hydrogenation of 1-dihydrocodeinone and ac U.S. C. 260-285 3 Claims 0. cording to a feature of the invention, there is therefore provided a process for the preparation of a purified 1-di ABSTRACT OF THE DESCLOSURE hydrocodeine which comprises the steps of A process for the preparation of a purified 1-dihydro (a) catalytically hydrogenating 1-dihydrocodeinone in a codeine which comprises the steps of: 5 liquid medium using a platinum oxide or supported (a) catalytically hydrogenating 1-dihydrocodeinone in a platinum metal catalyst to produce a solution of crude liquid medium using a catalyst selected from the group l-dihydrocodeine, consisting of platinum oxide and supported platinum (b) removing the catalyst from the solution, and metal to produce a solution of crude 1-dihydrocodeine, (c) treating the solution for the recovery of a purified (b) removing the catalyst from the solution, and 20 1-dihydrocodeine having a reduced content of l-dihy (c) treating the solution for recovery of a purified 1-di drothebainone and/or 1-dihydrothebainol. -

Australian Drug Law Reform Foundation
Can Australia respond to drugs more eFectively and safely? Roundtable report of law enforcement and other practitioners, researchers and advocates. Sydney, September 2015 Editors1 Roundtable Mick Palmer, report of Alex law enforcement Wodak, Bob and other Douglas practitioners, and Lyn researchers Stephens and advocates. Sydney, September 2015 “You are hamstrung by restrictions about what you can and cannot do. In alcohol prohibition in the United States the treatment of people with alcohol problems disappeared. You try and get treatment for your alcohol problem in Saudi Arabia today and it is not available. One of the negatives about drug prohibition when we see everything through a criminal justice lens is that drug treatment sufers. That is because it is treated as an adjunct to law enforcement rather than redefining the issue primarily as a health and social problem such as breast cancer, diabetes, high blood pressure. If we treated the drug problem as a health and social problem we would have new and better ways to manage it.” [Participant comment] Title for media purposes: “Can Australia respond to drugs more efectively and safely?” Australia21 Ltd ABN 25096242410 CAN 096242410 PO Box 3244, Weston, ACT 2611 Phone: 02 6288 0823 E-mail: [email protected] Web: www.australia21.org.au Design by Lester Bunnell, Paper Monkey ISBN: 978-0-9953842-1-7 Published: November 2016 Can Australia respond to drugs more eFectively and safely? Editors Mick Palmer, Alex Wodak, Bob Douglas and Lyn Stephens Roundtable report of law enforcement and other practitioners, researchers and advocates. Sydney, September 2015 While this report has been prepared for Australia21 in consultation with the participants, the views expressed do not reflect the views of all participants on every issue. -
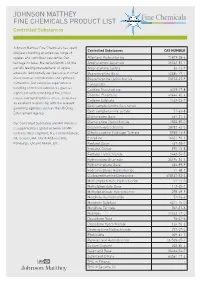
JOHNSON MATTHEY FINE CHEMICALS PRODUCT LIST Controlled Substances
JOHNSON MATTHEY FINE CHEMICALS PRODUCT LIST Controlled Substances Johnson Matthey Fine Chemicals has spent Controlled Substances CAS NUMBER 200 years building an extensive range of opiates and controlled substance. Our Alfentanil Hydrochloride 70879-28-6 heritage includes Macfarlan Smith Ltd, the Amphetamine Aspartate 25333-81-7 world’s leading manufacturer of opiate Amphetamine Sulfate 60-13-9 alkaloids. Additionally we specialise in other Buprenorphine Base 52485-79-7 areas such as cannabinoids and synthetic Buprenorphine Hydrochloride 53152-21-9 stimulants. Our extensive experience in Cannabidiol - handling controlled substances gives us Codeine Monohydrate 6059-47-8 significant understanding of the critical Codeine Phosphate 41444-62-6 issues surrounding these areas, as well as Codeine Sulphate 1420-53-7 an excellent relationship with the relevant Dextroamphetamine Saccharate - governing agencies such as the US Drug Dextroamphetamine Sulfate 51-63-8 Enforcement Agency. Diamorphine Base 561-27-3 Our Controlled Substance and API Portfolio Diamorphine Hydrochloride 1502-95-0 is supported by a global network of GMP Difenoxin Hydrochloride 28782-42-5 facilities: West Deptford, NJ; Conshohocken, Dihydrocodeine Hydrogen Tartrate 5965-13-9 PA; Devens, MA; North Andover, MA; Etorphine 14521-96-1 Edinburgh, UK and Annan, UK. Fentanyl Base 437-38-7 Fentanyl Citrate 990-73-8 Fentanyl Hydrochloride 1443-54-5 Hydrocodone Bitartrate 34195-34-1 Hydromorphone Base 466-99-9 Hydromorphone Hydrochloride 71-68-1 Lisdexamfetamine Dimesylate 608137-33-3 -

Government's Response to OFT's Review of Undertakings by Macfarlan Smith Limited
Government’s Response to OFT’s Review of Undertakings by MacFarlan Smith Limited (MSL) Market Analysis Opium in the UK – Analysis to date March 08 Sections in this report I) Background II) Home Office Undertaking III) Market Structure IV) Conclusions from Previous Market Analysis V) Taking the Analysis Forwards VI) Conclusions To Date Annex A: Copy of text from letter written by the EU Commission to EU Parliament re Opiates Annex B: Summary of responses to Home Office data request. I) Background a) OFT Review In 2006 the OFT published a report: Opium Derivatives, A Review of the Undertakings Given by Macfarlan Smith (MSL)1. This report recommended that the Government should consider competition when setting licensing policy for opium derivatives. b) Government Response The Department for Business, Enterprise and Regulatory Reform (BERR) has a general interest in OFT's market studies and co-ordinates Government responses where those studies make regulatory recommendations. Consideration of regulatory and policy issues is a matter for the relevant Department. In its response to the OFT2, the Government agreed to: Undertake to analyse the effects of MSL’s position as sole supplier on customers and competition, contrasting this with the positions of customers in other countries (both those who impose significant restrictions and those who do not). c) Work Undertaken by ERA & Drugs Liaison & Enforcement Unit (DLEU) BERR commissioned the Home Office to take forwards this market analysis. It was agreed that, at this stage, rather than repeat the OFT’s work a few years on (which was largely based on a survey of MSL’s UK customers) it made more sense to try and take the analysis forwards. -

Plan Mexico? Towards an Integrated Approach in the War on Drugs
SMALL WARS JOURNAL smallwarsjournal.com Plan Mexico? Towards an Integrated Approach in the War on Drugs by Alfonso Reyes The illegal drug trade has been present in Mexico since the beginning of the twentieth century when prohibition of the opium trade started. Since then, the social harm of the illegal drug trade in all its forms has been constantly increasing. Today, the most obvious example of the social harm of the illegal drug trade in Mexico is drug-related crime. As a result, Mexican authorities have launched a frontal attack against the drug cartels in an effort to reduce drug- related violence. However, the results of these efforts have not been as expected. One of the main problems that Mexican authorities face in their war on drugs is the lack of a well- coordinated anti-drug strategy to fight the illegal drug trade. Further, the efforts made by the Mexican government are based on a supply-reduction approach that has proved ineffective both in Mexico and around the world over the last century because it is not aimed at the social roots of the illegal drug trade. Thus, Mexico’s war on drugs has become a never-ending story. This thesis traces this history and then proposes a broader integrated approach based on attacking the roots of the illegal drug trade in Mexico. Part I: The “War on Drugs” in Mexico For more than ten years, the Mexican ment forces in the latter’s unsuccessful at- government has been following the same tempt to curtail or eliminate the illegal drug anti-drug policy in an effort to deter the il- trade.