The Separation of Low Density Polyethylene Laminates from Paper
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

FAQ About Recycling Cartons
FREQUENTLY ASKED QUESTIONS ABOUT CARTONS WHAT IS A CARTON? » Cartons are a type of packaging for food and beverage products you can purchase at the store. They are easy to recognize and are available in two types—shelf-stable and refrigerated. Shelf-stable cartons (types of products) Refrigerated (types of products) » Juice » Milk » Milk » Juice » Soy Milk » Cream » Soup and broth » Egg substitutes » Wine You will find these You will find these products in the chilled products on the shelves sections of grocery stores. in grocery stores. WHAT ARE CARTONS MADE FROM? » Cartons are mainly made from paper in the form of paperboard, as well as thin layers of polyethylene (plastic) and/or aluminum. Shelf-stable cartons contain on average 74% paper, 22% polyethylene and 4% aluminum. Refrigerated cartons contain about 80% paper and 20% polyethylene. ARE CARTONS RECYCLABLE? » Yes! Cartons are recyclable. In fact, the paper fiber contained in cartons is extremely valuable and useful to make new products. WHERE CAN I RECYCLE CARTONS? » To learn if your community accepts cartons for recycling, please visit RecycleCartons.com or check with your local recycling program. HOW DO I RECYCLE CARTONS? » Simply place the cartons in your recycle bin. If your recycling program collects materials as “single- stream,” you may place your cartons in your bin with all the other recyclables. If your recycling program collects materials as “dual-stream” (paper items together and plastic, metal and glass together), please place cartons with your plastic, metal and glass containers. WAIT, YOU JUST SAID CARTONS ARE MADE MAINLY FROM PAPER. Don’t I WANT TO PUT THEM WITH OTHER PAPER RECYCLABLES? » Good question. -

2021 Rain Barrel Program
2021 Rain Barrel Program This spring, LLCT and the Water Department/Water Commission are offering wholesale rain barrels through The Great American Rain Barrel Company (TGARBC). Order and pick up with LLCT’s Plant Kits on May 22nd from 8-11 A.M. at the DPW Site on Lewis Street. Water use has become an important issue in Lincoln. Recent years have seen drought conditions, and to protect the levels of our watershed, most summers and into fall residents have seen restrictions on outdoor water use. The largest residential water use is for watering lawns and gardens – according to the Lincoln Water Commission, our daily water use nearly doubles in the summer! Residents with wells might be surprised to learn that well water usage is also restricted, as that also drains the aquifer. There are many ways to conserve water use, and one ideal way to still provide water to necessary plants, including vegetable gardens, is to install rain barrels. Using rain barrels to water your gardens reduces the water drawn from our drinking water supply, allowing Lincoln to meet the DEP’s water conservation goals while simultaneously saving you money. That soft, chemical free water is very good for grass and other plants. During a rain storm, an enormous quantity of water runs off a roof, so you may want more than one! Each 4’ x 8’ section of roof that receives ¼” of rain will fill a 55-gallon barrel. TGARBC sells 100% re-purposed, food grade, UV protected and BPA free rain barrels. The barrels are produced in Massachusetts and will last for years when properly drained and stored for winter. -

Different Perspectives for Assigning Weights to Determinants of Health
COUNTY HEALTH RANKINGS WORKING PAPER DIFFERENT PERSPECTIVES FOR ASSIGNING WEIGHTS TO DETERMINANTS OF HEALTH Bridget C. Booske Jessica K. Athens David A. Kindig Hyojun Park Patrick L. Remington FEBRUARY 2010 Table of Contents Summary .............................................................................................................................................................. 1 Historical Perspective ........................................................................................................................................ 2 Review of the Literature ................................................................................................................................... 4 Weighting Schemes Used by Other Rankings ............................................................................................... 5 Analytic Approach ............................................................................................................................................. 6 Pragmatic Approach .......................................................................................................................................... 8 References ........................................................................................................................................................... 9 Appendix 1: Weighting in Other Rankings .................................................................................................. 11 Appendix 2: Analysis of 2010 County Health Rankings Dataset ............................................................ -

Corrugated Board Structure: a Review M.C
ISSN: 2395-3594 IJAET International Journal of Application of Engineering and Technology Vol-2 No.-3 Corrugated Board Structure: A Review M.C. Kaushal1, V.K.Sirohiya2 and R.K.Rathore3 1 2 Assistant Prof. Mechanical Engineering Department, Gwalior Institute of Information Technology,Gwalior, Assistant Prof. Mechanical Engineering 3 Departments, Gwalior Engineering College, Gwalior, M. Tech students Maharanapratap College of Technology, Gwalior, [email protected] [email protected] [email protected] ABSTRACT Corrugated board is widely used in the packing industry. The main advantages are lightness, recyclability and low cost. This makes the material the best choice to produce containers devoted to the shipping of goods. Furthermore examples of structure design based on corrugated boards can be found in different fields. Structural analysis of paperboard components is a crucial topic in the design of containers. It is required to investigate their strength properties because they have to protect the goods contained from lateral crushing and compression loads due to stacking. However in this paper complete and detailed information are presented. Keywords: - corrugated boards, recyclability, compression loads. Smaller flutes offer printability advantages as well as I. INTRODUCTION structural advantages for retail packaging. Corrugated board is essentially a paper sandwich consisting of corrugated medium layered between inside II. HISTORY and outside linerboard. On the production side, corrugated In 1856 the first known corrugated material was patented is a sub-category of the paperboard industry, which is a for sweatband lining in top hats. During the following four sub-category of the paper industry, which is a sub-category decades other forms of corrugated material were used as of the forest products industry. -
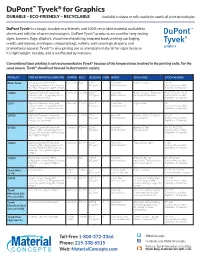
Tyvek Graphics Technical Data Sheet
DuPont ™ Tyvek ® for Graphics DURABLE – ECO-FRIENDLY – RECYCLABLE Available in sheets or rolls; usable for nearly all print technolo gies DuPont Tyvek a is a tough, durable, eco-friendly and 100% recyclable material available in sheets and rolls for all print technologies. DuPont Tyvek ® products are used for long-lasting signs, banners, flags, displays, visual merchandising, map and book printing, packaging, credit card sleeves, envelopes, shopping bags, wallets, wall coverings, drapery, and promotional apparel. Tyvek ® is also gaining use as a template material for signs because it is lightweight, durable, and is unaffected by moisture. Conventional laser printing is not recommended on Tyvek ® because of the temperatures involved in the printing units. For the same reason, Tyvek ® should not be used in electrostatic copiers. PRODUCT TYPE OF PRINTING USED FOR COATED MILS OZ. [GSM] CORE NOTES IDEAL USES STOCK WIDTHS Black Tyvek Flexography, Gravure, Offset Uncoated 5 mil 1.25 oz 2” Paper-like. Banners & Signs 36", 45" Lithography, Screen Process, UV-cure [42 gsm] Hard Structure Custom sizes available Inkjet (w/ testing due to lighter weight) Available in 10-yard rolls 1085D Digital on Demand, Flexography, Uncoated 10.3 mil 3.2 oz 3” Paper-like. Banners & Signs. Extra body 48.25", 57.125", 114.25" Gravure, Offset Lithography, Screen [109 gsm] Hard Structure for shape development. Custom sizes available Process, UV-cure Inkjet Available in 10-yard rolls 1079 Digital on Demand, Flexography, Uncoated 7.9 mil 2.85 oz 3” Paper-like. Tags & Labels 48" Gravure, Offset Lithography, Screen [97 gsm] Hard Structure Custom sizes available Process, Thermal Transfer, UV-cure Available in 10-yard rolls Inkjet 1073D Digital on Demand, Flexography, Uncoated 7.5 mil 2.2 oz 3” Paper-like. -

MEDIUM-DENSITY POLYETHYLENE YELLOW GAS Building Essentials MEETS ASTM D2513 for a Better Tomorrow MEDIUM-DENSITY POLYETHYLENE YELLOW GAS Meets ASTM D2513
MEDIUM-DENSITY POLYETHYLENE YELLOW GAS Building essentials MEETS ASTM D2513 for a better tomorrow MEDIUM-DENSITY POLYETHYLENE YELLOW GAS Meets ASTM D2513 II MEDIUM-DENSITY POLYETHYLENE YELLOW GAS MEDIUM-DENSITY POLYETHYLENE YELLOW GAS CONTENTS 01 PRODUCT DESCRIPTION .........................2 02 SHORT FORM SPECIFICATION. 6 03 DIMENSIONS AND WEIGHTS .......................7 04 SHORT FORM INSTALLATION GUIDE/WARNING . 9 05 WARRANTY ...................................10 MEDIUM-DENSITY POLYETHYLENE YELLOW GAS 1 01 PRODUCT DESCRIPTION MEDIUM-DENSITY POLYETHYLENE YELLOW GAS FOR FUEL GAS USE IN MULTIPLE APPLICATIONS FOR GAS DISTRIBUTION DESCRIPTION QUALITY CONTROL Polyethylene gas pipes are the preferred natural gas distri- JM Eagle™ takes great pride in the quality and workman- bution piping product of choice with over 90% usage in ship of all of our products. JM Eagle™ quality control pro- North America today. Polyethylene gas pipes are light- grams encompass three critical aspects of the manufac- weight, non-corrosive, available in coil lengths, and easy turing process: the incoming raw material, pipe produc- to install by heat fusion or mechanical fittings. For these tion, and the finished goods. Incoming material is visually reasons, PE pipes have been proven reliable, durable, and inspected and tested to ensure the material meets all have been in use since the 1960’s. applicable requirements before its release for production. During production, the pipe will be visually examined for any cosmetic defect and pipe samples will be collected for physical verification and testing for compliance. The finished product is subjected to further visual inspection to ensure it has met all the appropriate specifications and packaging requirements. Without exception, our pipes are constantly monitored throughout the entire manufac- turing process to validate that they are in accordance with all applicable specifications. -

A Quest for the Golden Fleece
A Quest for the Golden Fleece Donald Farnsworth Copyright © 2017 Donald Farnsworth, all rights reserved. Any person is hereby authorized to view, copy, print and distribute this document for informational and non-commercial purposes only. Any copy of this document or portion thereof must include this copyright notice. Note that any product or technology described in the document may be the subject of other intellectual property rights reserved by Don- ald Farnsworth and Magnolia Editions or other entities. 2 A Quest for the Golden Fleece Rarely have I encountered an entangled mat of cellulose fibers I didn’t appreciate in one way or another. Whether textured or smooth, pre- cious or disposable, these hardy amalgams of hydrogen-bonded fibers have changed the world many times over. For centuries, human history has been both literally written on the surface of paper and embedded deep within its structure. In folded and bound form, mats of cellulose fibers ushered in the Enlightenment; by enabling multiple iterations and revisions of an idea to span generations, they have facilitated the design of airships and skyscrapers, or the blueprints and calculations that made possible the first human footsteps on the moon. Generally speaking, we continue to recognize paper by a few basic characteristics: it is most often thin, portable, flexible, and readily ac- cepting of ink or inscription. There is, however, a particular sheet that is superlatively impressive, exhibiting an unintentional and unpreten- tious type of beauty. At first glance and in direct light, it may trick you into thinking it is merely ordinary paper; when backlit, the care- ful viewer may detect subtle hints of its historical pedigree via telltale watermarks or chain and laid lines. -

21 Tips for Troubleshooting Shrinkwrap & Equipment
Help for Diagnosing Shrink Packaging’s Leading Profit Busters From bad seals to burn-through, this handy guide It’s all part of our commitment to deliver shrink helps you address shrink packaging’s most common packaging’s best answers…the best experts…and the film/equipment issues. best bottom line. But don’t feel like you need to go it alone! Clysar SHRINK HELP HOTLINE: distributors and their trained field service technicians 1-888-4-Clysar are available 24/7 to support you with unrivaled Clysar supports distributors technical expertise. Call them to: and customers with technical • Troubleshoot reoccurring operating problems field specialists located and turn them into cost-saving solutions. throughout the country. • Transform profit-eating rejects into beautiful These knowledgeable display packages. advisors have years of experience troubleshooting • Fast-track new brand packages or film/equipment in thousands of shrink start-ups. operations, and are available • Add speed or capabilities to your existing shrink to provide technical operation. consultations via phone or • Improve uptime with preventive maintenance, on-site. training and more. WHAT TO CHECK WHEN GOOD SEALS GO BAD IS THE PROBLEM THE MACHINE OR THE PACKAGE? TIP #I: TIP #2: Start with the Do the One-Minute Big Three Seal Test 99% of all shrink packaging problems come down to three basic issues: 1. Temperature (too hot/cool) 2. Time (too much/little) 3. Pressure (incorrect seal pressure and tension) If you can correct these shrink fundamentals, you’re on your way to beautiful, trouble-free packages. The following tips give you more details on specific Here’s a quick test to help you determine if a bad actions you can take. -

What Is a Rain Barrel?
What is a rain barrel? A rain barrel is a container used to collect and store rainwater from your roof that would otherwise be lost to runoff and diverted out onto your property or to a storm drain and eventually to local streams or rivers. Rain barrels are also an economical way to store rain water to be used as a secondary water supply for indoor plants, flower gardens, lawns, fill the bird bath, and washing cars and windows. Rain barrels are usually about 40-60 gallons and can be purchased or made relatively easily. The parts are available at any hardware store. *Stored water is not used for drinking or bathing* Why use rain barrels? Every time it rains, unabsorbed water rushes to storm drains and directly into our local waterways. Often times this runoff carries with it pollutants it has picked up along the way depositing in them into local waterways. Any rainwater in an urban or suburban area that does not evaporate or infiltrate into the ground is considered stormwater. Infiltration is when water on the ground surface soaks into the soil. Impervious surfaces like roofs, asphalt, and concrete do not allow Rain water from your roof and driveway travels to the street and into storm drains for the infiltration to occur. eventually draining into our creeks, lakes, and rivers. Infiltration of water on pervious surfaces is important because it reduces the amount runoff and the possibility of erosion and pollutants leaving a site and entering a waterway. What can rain barrels do for you? Healthier plants. -

Expanded Polystyrene Food Service Take-Out Container Study
Appendix 1.1. California Cities that have Pursued a Polystyrene Ban Please note that not all of these bans are in place: many have been challenged or overturned. Alameda (2008) Expanded polystyrene ban, requirement that all takeout food packaging be compostable or recyclable Albany (2008) Expanded polystyrene ban, requirement that all takeout food packaging be compostable or recyclable Aliso Viejo (2005) Government facility expanded polystyrene ban Ordinance #2004-060 Berkeley (adopted 1988) Expanded polystyrene ban, requirement that 50% of takeout food packaging be recyclable or compostable Title 11.58 and 11.60 of Municipal Code Calabasas (2008) Expanded polystyrene ban, requirement that all takeout food packaging be recyclable or compostable Capitola (2009) Expanded polystyrene ban, requirement that all disposable takeout food packaging be compostable Carmel (1989) Expanded polystyrene ban, requirement that 50% of takeout food packaging be recyclable, compostable or reusable Del Ray Oaks (effective July 1, 2010) Expanded polystyrene ban, requirement that all takeout food packaging More information available on be recyclable or compostable page 35 of Agenda Packet Emeryville (2008) Expanded polystyrene ban, requirement that all takeout food packaging be recyclable or compostable Fairfax (1993) Expanded polystyrene ban for all restaurants and food retail vendors Title 8.16 of Municipal Code Fremont (effective January 1, 2011) Expanded polystyrene ban for food vendors, requirement that all takeout food packaging be recyclable or compostable Appendix 1.1 | i Hayward (effective July 2011) Expanded polystyrene ban for restaurant vendors, requirement that takeout food packaging be recyclable or compostable Hercules (2008) Expanded polystyrene ban Sec. 5-3109, Title 5, Chapter 3 of Municipal Code Huntington Beach (2005) Government facility expanded polystyrene ban Laguna Beach (2008) Polystyrene ban, requirement that all plastic takeout food packaging be recyclable Title 7. -

Label Liners: Meeting the Sustainability Challenge
Label liners: meeting the sustainability challenge By Mariya Nedelcheva, Product Manager Film & With ever-greater emphasis placed on packaging and waste reduction, Jenny Wassenaar, Compliance & Sustainability brand owners are looking for solutions that secure their sustainability Director, Avery Dennison credentials. Consumers pay particularly close attention to packaging, where there are significant environmental gains to be made. For example, waste from labelling can create useful by-products and circular economies. Waste is not always visible on the final packaging, but its impact on brand reputation is no less real. Consumers’ perceptions of a brand can be enhanced when sustainability is improved. label.averydennison.com Matrix Liner Final White paper waste waste label 16% 35% 37% Start-up waste plus Challenge printed End-user The challenge of recycling waste from the labelling process in error scrap - and ideally creating useful by-products - is complex. Many 10% 1% different elements must be addressed in order to move towards the ultimate goal of zero waste. For example, the word ‘recyclable’ can mean many things, and should not be viewed in isolation. Today there is a chance that recyclable products will still end up in landfill, so what matters is establishing genuinely viable end-to-end recycling solutions. That means considering every component within packaging, including where it comes from, how much material has been used, and what happens at every stage of the package’s journey through the value chain. This paper discusses how the sustainability of labelling laminates can be improved, with a particular focus on the label release liners that are left behind once labels have been dispensed. -

DID YOU KNOW? PET (Polyethylene Terephthalate) Is Actually Polyester. When PET Is Used for Bottles, Containers and Other
355 Lexington Ave., Suite 1500 ▪ New York, NY 10017 ▪ www.PETresin.org DID YOU KNOW? Little-Known Facts about PET Plastic . PET (polyethylene terephthalate) is actually polyester. When PET is used for bottles, containers and other applications, it is called PET or PET resin. When PET is used as a fiber, it is typically called polyester. The PET bottle was invented by Nathaniel C. Wyeth, a DuPont engineer and brother of American painter Andrew Wyeth. The patent was issued to Wyeth in 1973 and assigned to DuPont. According to the EPA, recycling one pound of PET bottles (that’s about 10 two-liter soda bottles) saves approximately 26,000 BTUs of energy. PET bottles and the sun are helping millions of people in developing countries obtain potable water. Using a system called SODIS (solar water disinfection), inhabitants set water-filled PET bottles in the sun for several hours or days – depending on how much sunlight is available – as a simple but effective means of destroying disease-causing bacteria and gaining safe drinking water. More than 1.5 billion pounds of used PET bottles and containers are collected in the U.S. each year for recycling. PET is the most recycled plastic in the U.S. and the world. A single-serve PET bottle (0.5 liter) is strong enough to hold 50 times its weight in water. Chemists keep finding new ways to make PET lighter without losing any strength. A 2-liter PET bottle that weighed 68 grams in 1980 now weighs as little as 42 grams. The average weight of single-serve 0.5 liter PET water bottle is now 9.9 grams, nearly half of what it weighed in 2000.