Morphological Descriptions of Laboratory Reared Larvae And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

U , '' Regional (;"Ntre of I:::;~, I Central Marine Fisheries Research Institute T - ~ '\~ ~ ~3L'!" ICAR Marine Fisheries P.O
CMFRI S ammelt S ,4(J(Jt (JU Recent Advances in Se"ed Production and Growout Techniques for Marine Finfish and Shellfish ,1 I Compiled and Edited by Dr. G.Gopakumar, Principal Scientist & Director, Summer School and '. Dr. Boby Ignatius, Scientist (SS) Central Marine Fisheries Research Institute j' U , '' Regional (;"ntre of i:::;~, I Central Marine Fisheries Research Institute t - ~ '\~ ~ ~3l'!" ICAR Marine Fisheries P.O . Tamil Nadu - 623 520 ' ~~ ... ~"'~~ SEED PRODUCTION OF THE SAND LOBSTER THENUS ORIENTALIS (LUND) Joe K. Kizhakudan Research Centre of CMFRI, Chennai 3f With the decline in many commercial fisheries worldwide and an ever increasing demand for seafood protein, there is a growing need for augmenting the production of high-protein, high-value resources like lobsters. Aquaculture remains the ideal measure to augment production and ensure conseNation, and even enhancement. of natural stocks. Aquaculture provides a two-pronged solution towards increasing the fish production through ., farming of hatchery-produced seed of commercially important finfishes and shellfishes , enhancing natural stocks by sea ranching hatchery-produced seed of commercially important finfishes and shellfishes Lobsters are among the most priced seafood delicacies enjoying a special demand in international markets. As against a world average annual productio'n of2.1 lakh tonnes, India's average annual lobster production is about 2000 tonnes. With the distinction of being perhaps, the only seafood resource in India's trade economy, which remains relatively low down the ladder in terms of quantity of production but brings in maximum foreign exchange, lobsters have been the subject of study for more than two decades now. -

Lobsters-Identification, World Distribution, and U.S. Trade
Lobsters-Identification, World Distribution, and U.S. Trade AUSTIN B. WILLIAMS Introduction tons to pounds to conform with US. tinents and islands, shoal platforms, and fishery statistics). This total includes certain seamounts (Fig. 1 and 2). More Lobsters are valued throughout the clawed lobsters, spiny and flat lobsters, over, the world distribution of these world as prime seafood items wherever and squat lobsters or langostinos (Tables animals can also be divided rougWy into they are caught, sold, or consumed. 1 and 2). temperate, subtropical, and tropical Basically, three kinds are marketed for Fisheries for these animals are de temperature zones. From such partition food, the clawed lobsters (superfamily cidedly concentrated in certain areas of ing, the following facts regarding lob Nephropoidea), the squat lobsters the world because of species distribu ster fisheries emerge. (family Galatheidae), and the spiny or tion, and this can be recognized by Clawed lobster fisheries (superfamily nonclawed lobsters (superfamily noting regional and species catches. The Nephropoidea) are concentrated in the Palinuroidea) . Food and Agriculture Organization of temperate North Atlantic region, al The US. market in clawed lobsters is the United Nations (FAO) has divided though there is minor fishing for them dominated by whole living American the world into 27 major fishing areas for in cooler waters at the edge of the con lobsters, Homarus americanus, caught the purpose of reporting fishery statis tinental platform in the Gul f of Mexico, off the northeastern United States and tics. Nineteen of these are marine fish Caribbean Sea (Roe, 1966), western southeastern Canada, but certain ing areas, but lobster distribution is South Atlantic along the coast of Brazil, smaller species of clawed lobsters from restricted to only 14 of them, i.e. -
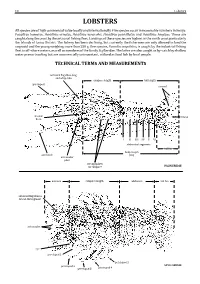
Lobsters LOBSTERS§
18 Lobsters LOBSTERS§ All species are of high commercial value locally and internationally. Five species occur in reasonable numbers in Kenya: Panulirus homarus, Panulirus ornatus, Panulirus versicolor, Panulirus penicillatus and Panulirus longipes. These are caughtungravid along and the the coast young by weighing the artisanal more fishing than 250 fleet. g. Landings One species, of these Puerulus species angulatus are highest in the north coast particularly the Islands of Lamu District. The fishery has been declining,Scyllaridae. but currently The latter the fishermen are also caught are only as by–catch allowed toby landshallow the , is caught by the industrial fishing fleet in off–shore waters, as well as members of the family water prawn trawling but areTECHNICAL commercially unimportant, TERMS AND utilized MEASUREMENTS as food fish by local people. and whip–like antennal flagellum long carapace length tail length pereiopod uropod frontal telson horn III III IV VIV abdominal segments tail fan body length antennule (BL) antennular plate strong spines on carapace PALINURIDAE antenna carapace length abdomen tail fan antennal flagellum a broad, flat segment antennules eye pereiopod 1 pereiopod 5 pereiopod 2 SCYLLARIDAE pereiopod 3 pereiopod 4 Guide to Families 19 GUIDE TO FAMILIES NEPHROPIDAE Page 20 True lobsters § To about 15 cm. Marine, mainly deep waters on soft included in the Guide to Species. 1st pair of substrates. Three species of interest to fisheriespereiopods are large 3rd pair of pereiopods with chela PALINURIDAE Page 21 Antennal Spiny lobsters § To about 50 cm. Marine, mostly shallow waters on flagellum coral and sand stone reefs, some species on soft included in the Guide to Species. -

DOCTORAL THESIS a Physio-Ecological Study of Ephyrae
DOCTORAL THESIS A Physio-ecological Study of Ephyrae of the Common Jellyfish Aurelia aurita s.l. (Cnidaria: Scyphozoa), with Special Reference to their Survival Capability under Starvation Zhilu Fu Graduate School of Biosphere Science Hiroshima University September 2014 DOCTORAL THESIS A Physio-ecological Study of Ephyrae of the Common Jellyfish Aurelia aurita s.l. (Cnidaria: Scyphozoa), with Special Reference to their Survival Capability under Starvation Zhilu Fu Department of environmental Dynamics and Management Graduate School of Biosphere Science Hiroshima University September 2014 Abstract The moon jellyfish Aurelia aurita s.l. is the most common scyphozoan jellyfish in the coastal waters around the world, and the mass occurrences of this species have been reported from various regions. In recent decades, A. aurita blooms have become increasingly prominent in East Asian seas, causing serious problems to human sectors such as fisheries and coastal power plant operations. Therefore, it is important to identify causes for the enhancement of A. aurita populations to forecast likely outbreaks prior to the season of medusa blooms. In the population dynamics of scyphozoan jellyfish, the following two factors are important to determine the size of adult (medusa) population: (1) the abundance of benthic polyps, which reproduce asexually and undergo seasonal strobilation to release planktonic ephyrae, and (2) the mortality of ephyrae before recruitment to the medusa stage. Although much knowledge has been accumulated about physio-ecology of the polyp stage by previous studies, only few studies have been conducted for the ephyra stage. The success for survival through larval stage is basically affected by two factors, viz. food availability and predation. -

Multiple Measures of Thermal Performance of Early Stage Eastern Rock Lobster in a Fast-Warming Ocean Region
Vol. 624: 1–11, 2019 MARINE ECOLOGY PROGRESS SERIES Published August 15 https://doi.org/10.3354/meps13054 Mar Ecol Prog Ser OPENPEN ACCESSCCESS FEATURE ARTICLE Multiple measures of thermal performance of early stage eastern rock lobster in a fast-warming ocean region Samantha Twiname1,*, Quinn P. Fitzgibbon1, Alistair J. Hobday2, Chris G. Carter1, Gretta T. Pecl1 1Institute for Marine and Antarctic Studies, University of Tasmania, Private Bag 49, Hobart, TAS 7001, Australia 2CSIRO Oceans and Atmosphere, Castray Esplanade, Hobart, TAS 7001, Australia ABSTRACT: To date, many studies trying to under- stand species’ climate-driven changes in distribution, or ‘range shifts’, have each focused on a single poten- tial mechanism. While a single performance measure may give some insight, it may not be enough to ac - curately predict outcomes. Here, we used multiple measures of performance to explore potential mech- anisms behind species range shifts. We examined the thermal pattern for multiple measures of performance, including measures of aerobic metabolism and multi- ple as pects of escape speed, using the final larval stage (puerulus) of eastern rock lobster Sagmariasus verreauxi as a model species. We found that aerobic scope and escape speed had different thermal per- Single thermal performance measures for eastern rock lob- formances and optimal temperatures. The optimal ster Sagmariasus verreauxi may not accurately predict whole temperature for aerobic scope was 27.5°C, while the animal performance under future ocean warming. pseudo-optimal temperature for maximum escape Photo: Peter Mathew speed was 23.2°C. This discrepancy in thermal per- formance indicators illustrates that one measure of performance may not be sufficient to accurately pre- 1. -

Does Climate Change Bolster the Case for Fishery Reform in Asia? Christopher Costello∗
Does Climate Change Bolster the Case for Fishery Reform in Asia? Christopher Costello∗ I examine the estimated economic, ecological, and food security effects of future fishery management reform in Asia. Without climate change, most Asian fisheries stand to gain substantially from reforms. Optimizing fishery management could increase catch by 24% and profit by 34% over business- as-usual management. These benefits arise from fishing some stocks more conservatively and others more aggressively. Although climate change is expected to reduce carrying capacity in 55% of Asian fisheries, I find that under climate change large benefits from fishery management reform are maintained, though these benefits are heterogeneous. The case for reform remains strong for both catch and profit, though these numbers are slightly lower than in the no-climate change case. These results suggest that, to maximize economic output and food security, Asian fisheries will benefit substantially from the transition to catch shares or other economically rational fishery management institutions, despite the looming effects of climate change. Keywords: Asia, climate change, fisheries, rights-based management JEL codes: Q22, Q28 I. Introduction Global fisheries have diverged sharply over recent decades. High governance, wealthy economies have largely adopted output controls or various forms of catch shares, which has helped fisheries in these economies overcome inefficiencies arising from overfishing (Worm et al. 2009) and capital stuffing (Homans and Wilen 1997), and allowed them to turn the corner toward sustainability (Costello, Gaines, and Lynham 2008) and profitability (Costello et al. 2016). But the world’s largest fishing region, Asia, has instead largely pursued open access and input controls, achieving less long-run fishery management success (World Bank 2017). -

Goldstein Et Al 2019
Journal of Crustacean Biology Advance Access published 24 August 2019 Journal of Crustacean Biology The Crustacean Society Journal of Crustacean Biology 39(5), 574–581, 2019. doi:10.1093/jcbiol/ruz055 Downloaded from https://academic.oup.com/jcb/article-abstract/39/5/574/5554142/ by University of New England Libraries user on 04 October 2019 Development in culture of larval spotted spiny lobster Panulirus guttatus (Latreille, 1804) (Decapoda: Achelata: Palinuridae) Jason S. Goldstein1, Hirokazu Matsuda2, , Thomas R. Matthews3, Fumihiko Abe4, and Takashi Yamakawa4, 1Wells National Estuarine Research Reserve, Maine Coastal Ecology Center, 342 Laudholm Farm Road, Wells, ME 04090 USA; 2Mie Prefecture Fisheries Research Institute, 3564-3, Hamajima, Shima, Mie 517-0404 Japan; 3Florida Fish and Wildlife Conservation Commission, Fish and Wildlife Research Institute, 2796 Overseas Hwy, Suite 119, Marathon, FL 33050 USA; and 4Department of Aquatic Bioscience, Graduate School of Agricultual and Life Sciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo, Tokyo 113-8657 Japan HeadA=HeadB=HeadA=HeadB/HeadA Correspondence: J.S. Goldstein: e-mail: [email protected] HeadB=HeadC=HeadB=HeadC/HeadB (Received 15 May 2019; accepted 11 July 2019) HeadC=HeadD=HeadC=HeadD/HeadC Ack_Text=DisHead=Ack_Text=HeadA ABSTRACT NList_lc_rparentheses_roman2=Extract1=NList_lc_rparentheses_roman2=Extract1_0 There is little information on the early life history of the spotted spiny lobster Panulirus guttatus (Latreille, 1804), an obligate reef resident, despite its growing importance as a fishery re- BOR_HeadA=BOR_HeadB=BOR_HeadA=BOR_HeadB/HeadA source in the Caribbean and as a significant predator. We cultured newly-hatched P. guttatus BOR_HeadB=BOR_HeadC=BOR_HeadB=BOR_HeadC/HeadB larvae (phyllosomata) in the laboratory for the first time, and the growth, survival, and mor- BOR_HeadC=BOR_HeadD=BOR_HeadC=BOR_HeadD/HeadC phological descriptions are reported through 324 days after hatch (DAH). -

The Biology and Fisheries of the Slipper Lobster
The Biology and Fisheries of the Slipper Lobster Kari L. Lavalli College of General Studies Boston University Bo~ton, Massachusetts, U.S.A. Ehud Spanier The Leon Recanati Institute for Maritime Studies and Department of Maritime Civilizations University of Haifa Haifa, Israel 0 ~y~~F~~~~~oup Boca Raton London New York CRC Press is an imprint of the Taylor & Francis Group, an informa business Cover image courtesy of Megan Elizabeth Stover of the College of General Studies, Boston University, Boston, Massachusetts. CRC Press Taylor & Francis Group 6000 Broken Sound Parkway NW, Suite 300 Boca Raton, FL 33487-2742 © 2007 by Taylor & Francis Group, LLC CRC Press is an imprint of Taylor & Francis Group, an Informa business No claim to original U.S. Government works Printed in the United States of America on acid-free paper 10 9 8 7 6 5 4 3 2 1 International Standard Book Number-10: 0-8493-3398-9 (Hardcover) International Standard Book Number-13: 978-0-8493-3398-9 (Hardcover) This book contains information obtained from authentic and highly regarded sources. Reprinted material is quoted with permission, and sources are indicated. A wide variety of references are listed. Reasonable efforts have been made to publish reliable data and information, but the author and the publisher cannot assume responsibility for the validity of all materials or for the consequences of their use. No part ofthis book may be reprinted, reproduced, transmitted, or utilized in any form by any electronic, mechanical, or other means, now known or hereafter invented, including photocopying, microfilming, and recording, or in any informa tion storage or retrieval system, without written permission from the publishers. -

Large Spiny Lobsters Reduce the Catchability of Small Conspecifics
Vol. 666: 99–113, 2021 MARINE ECOLOGY PROGRESS SERIES Published May 20 https://doi.org/10.3354/meps13695 Mar Ecol Prog Ser OPEN ACCESS Size matters: large spiny lobsters reduce the catchability of small conspecifics Emma-Jade Tuffley1,2,3,*, Simon de Lestang2, Jason How2, Tim Langlois1,3 1School of Biological Sciences, The University of Western Australia, 35 Stirling Highway, Crawley, WA 6009, Australia 2Aquatic Science and Assessment, Department of Primary Industries and Regional Development, 39 Northside Drive, Hillarys, WA 6025, Australia 3The UWA Oceans Institute, Indian Ocean Marine Research Centre, Cnr. of Fairway and Service Road 4, Crawley, WA 6009, Australia ABSTRACT: Indices of lobster abundance and population demography are often derived from pot catch rate data and rely upon constant catchability. However, there is evidence in clawed lobsters, and some spiny lobsters, that catchability is affected by conspecifics present in pots, and that this effect is sex- and size-dependent. For the first time, this study investigated this effect in Panulirus cyg nus, an economically important spiny lobster species endemic to Western Australia. Three studies: (1) aquaria trials, (2) pot seeding experiments, and (3) field surveys, were used to investi- gate how the presence of large male and female conspecifics influence catchability in smaller, immature P. cygnus. Large P. cygnus generally reduced the catchability of small conspecifics; large males by 26−33% and large females by 14−27%. The effect of large females was complex and varied seasonally, dependent on the sex of the small lobster. Conspecific-related catchability should be a vital consideration when interpreting the results of pot-based surveys, especially if population demo graphy changes. -

REMINDER: Supporting Seafood Future (SSF) Grants Program
PFA Update 4 January 2019 The PFA represents its members’ interests. If you need our help on any issue, please do not hesitate in contacting the PFA head office (6652-7374) &/or the Chief Executive Officer (0429303371) REMINDER: Supporting Seafood Future (SSF) grants program The Supporting Seafood Future (SSF) grants program is designed to build marketing and promotion capability within seafood businesses through small-scale (less than $10,000 including GST) and large-scale ($10,000 to $200,000 including GST) grants. The purpose of the campaign is to increase consumption of NSW seafood, drive the value of NSW seafood through increased awareness and consumption, and build industry capabilities and cohesiveness. Strategic objectives of the campaign are to: • Increase value, through consumption of farmed and wild caught NSW seafood • Increase shopper options across a broader variety of NSW seafood • Drive awareness and build social licence of the NSW seafood community Contact the PFA if you have any ideas that you wish our support, partnership or would like us to help put together an application. Information regarding program guidelines are outlined in the Program Guidelines. NATIONAL SEAFOOD INDUSTRY LEADERSHIP PROGRAM (NSILP 2019) Applications are now open for the 2019 National Seafood Industry Leadership Program. Each year the Sydney Fish Market is proud to fund 2-3 staff/industry representatives to attend the program. If you would like to be put forward by SFM as one of their sponsored candidates, please contact SFM or the PFA for the nomination form. These forms must be returned to SFM by Friday Jan 11th. The National Seafood Industry Leadership Program (NSLIP) is designed for people wishing to take up leadership roles throughout the industry. -

Ictalurus Punctatus) Female X Blue Catfish (Ictalurus Furcatus) Male Hybrid Embryos
Studies for Improvement of Reproductive Biotechnology for Production of Channel Catfish (Ictalurus punctatus) Female X Blue Catfish (Ictalurus furcatus) Male Hybrid Embryos by Dayan Anselm Perera A dissertation submitted to the Graduate Faculty of Auburn University in partial fulfillment of the requirements for the Degree of Doctor of Philosophy Auburn, Alabama December 8, 2012 Keywords: Efficacy, Toxicity, OVA-EAZE, LHRHa, Xenogenics Copyright 2012 by Dayan A. Perera Approved by Rex A. Dunham, Chair, Alumni Professor, Fisheries and Allied Aquacultures Ronald P. Phelps, Associate Professor, Fisheries and Allied Aquacultures Eric J. Peatman, Assistant Professor, Fisheries and Allied Aquacultures Joseph C. Newton Associate professor, College of Veterinary Medicine Abstract Investigative studies were conducted on the relative effectiveness between carp pituitary extract (CPE), luteinizing hormone releasing hormone analog (LHRHa) injections and LHRHa implants for producing hybrid catfish embryos. Data from the past 15 years, which included, 25 on CPE, 20 on LHRHa injections, and 20 for LHRHa implants, respectively, were evaluated. LHRHa administered as an injection or implant produced more (P<0.001) fry/kg than CPE. Mean fry/kg female body weight (all females) produced was 948 for, CPE 2,483 LHRHa injections and 2,765 for LHRHa. There was not a significant difference in fry/kg between the two LHRHa treatments. The coefficient of variance indicated more consistent results for CPE (CV=37.5), and LHRHa implants (CV= 35.9) than LHRHA injections (CV= 49.9). The second study investigated the effectiveness of OVA-EAZE a Luteinizing Hormone Releasing Hormone analog (LHRHa). This study investigated des-Gly10,[D-Ala6] LHRH Ethyl amide (LHRHa) administered in two doses of 20 µg per kilogram of female channel catfish body weight as a priming dose, followed 12 hours later by a resolving injection of 100 µg per kilogram of body weight, to determine its effectiveness in inducing ovulation in channel catfish (Ictalurus punctatus). -

Developmental Changes in the Mouthparts of Juvenile Caribbean Spiny Lobster, Panulirus Argus: Implications for Aquaculture
FAD LIBRARIES Florida Atlantic University HARBOR BRANCH ~ FLORIDA ATLANTIC UNIVERSITY FAU Institutional Repository http://purl.fcla.edu/fau/fauir This paper was submitted by the faculty of FAU’s Harbor Branch Oceanographic Institute. Notice: ©2008 Elsevier B.V. The final published version of this manuscript is available at http://www.sciencedirect.com/science/journal/00448486. This article may be cited as: Cox, S. L., Jeffs, A. G., & Davis, M. (2008). Developmental changes in the mouthparts of juvenile Caribbean spiny lobster, Panulirus argus: Implications for aquaculture. Aquaculture, 283(1‐4), 168‐174. doi:10.1016/j.aquaculture.2008.07.019 Aquaculture 283 (2008) 168–174 Contents lists available at ScienceDirect Aquaculture journal homepage: www.elsevier.com/locate/aqua-online Developmental changes in the mouthparts of juvenile Caribbean spiny lobster, Panulirus argus: Implications for aquaculture Serena L. Cox a,b,⁎, Andrew G. Jeffs c, Megan Davis a a Aquaculture Division, Harbor Branch Oceanographic Institute at Florida Atlantic University, 5600 US 1 North, Fort Pierce, Florida 34946, USA b National Institute of Water and Atmospheric Research, Private Bag 14901, Kilbirnie, Wellington 6241, New Zealand c Leigh Marine Laboratory, University of Auckland, P.O. Box 349, Warkworth 0941, New Zealand article info abstract Article history: Light microscopy and video analysis were used to examine the mouthpart morphology and feeding Received 5 May 2008 behaviour of the Caribbean spiny lobster from puerulus (megalopal stage) (5–8 mm carapace length, CL) to Received in revised form 14 July 2008 adult (85 mm CL). Upon settlement the pueruli did not possess fully functional mouthparts, however, Accepted 14 July 2008 efficient feeding appendages appeared in the first instar juvenile (after the first moult from puerulus).