Structure and Function of the Feeding Apparatus in the Common Musk Turtle Sternotherus Odoratus (Chelonia, Kinosternidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Ecology and Evolutionary History of Two Musk Turtles in the Southeastern United States
The University of Southern Mississippi The Aquila Digital Community Dissertations Spring 2020 The Ecology and Evolutionary History of Two Musk Turtles in the Southeastern United States Grover Brown Follow this and additional works at: https://aquila.usm.edu/dissertations Part of the Genetics Commons Recommended Citation Brown, Grover, "The Ecology and Evolutionary History of Two Musk Turtles in the Southeastern United States" (2020). Dissertations. 1762. https://aquila.usm.edu/dissertations/1762 This Dissertation is brought to you for free and open access by The Aquila Digital Community. It has been accepted for inclusion in Dissertations by an authorized administrator of The Aquila Digital Community. For more information, please contact [email protected]. THE ECOLOGY AND EVOLUTIONARY HISTORY OF TWO MUSK TURTLES IN THE SOUTHEASTERN UNITED STATES by Grover James Brown III A Dissertation Submitted to the Graduate School, the College of Arts and Sciences and the School of Biological, Environmental, and Earth Sciences at The University of Southern Mississippi in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Approved by: Brian R. Kreiser, Committee Co-Chair Carl P. Qualls, Committee Co-Chair Jacob F. Schaefer Micheal A. Davis Willian W. Selman II ____________________ ____________________ ____________________ Dr. Brian R. Kreiser Dr. Jacob Schaefer Dr. Karen S. Coats Committee Chair Director of School Dean of the Graduate School May 2020 COPYRIGHT BY Grover James Brown III 2020 Published by the Graduate School ABSTRACT Turtles are among one of the most imperiled vertebrate groups on the planet with more than half of all species worldwide listed as threatened, endangered or extinct by the International Union of the Conservation of Nature. -

Bite-Force Generation and Feeding Biomechanics in the Loggerhead Musk Turtle, Sternotherus Minor: Implications for the Ontogeny of Performance Joseph Bryce Pfaller
Florida State University Libraries Electronic Theses, Treatises and Dissertations The Graduate School 2009 Bite-Force Generation and Feeding Biomechanics in the Loggerhead Musk Turtle, Sternotherus Minor: Implications for the Ontogeny of Performance Joseph Bryce Pfaller Follow this and additional works at the FSU Digital Library. For more information, please contact [email protected] FLORIDA STATE UNIVERISTY COLLEGE OF ARTS AND SCEINCES BITE-FORCE GENERATION AND FEEDING BIOMECHANICS IN THE LOGGERHEAD MUSK TURTLE, STERNOTHERUS MINOR: IMPLICATIONS FOR THE ONTOGENY OF PERFORMANCE By JOSEPH BRYCE PFALLER A Thesis submitted to the Department of Biological Science in partial fulfillment of the requirements for the degree of Master of Science Degree Awarded: Spring Semester, 2009 The members of the Committee approve the Thesis of Joseph Bryce Pfaller defended on April 8th, 2009. ______________________________ Gregory M. Erickson Professor Directing Thesis ______________________________ Brian D. Inouye Committee Member ______________________________ William S. Oates Committee Member Approved: ________________________________________________________________________ P. Bryant Chase, Chair, Department of Biological Science The Graduate School has verified and approved the above named committee members. ii For my parents, Michael Pfaller and Beverly Ringenberg and my brother, Stephen Pfaller iii ACKNOWLEDGEMENTS First and foremost, I would like to sincerely thank Paul Gignac. Paul has been integral to my development as a scientist during my time at Florida State. He was always available for thought-provoking discussions and never hesitated to assist me when I sought help. He provided extensive statistical assistance and was vital to the success of the mechanical loading tests. Paul has been a great friend and colleague to me while at Florida State, and I thank him immensely. -

In AR, FL, GA, IA, KY, LA, MO, OH, OK, SC, TN, and TX): Species in Red = Depleted to the Point They May Warrant Federal Endangered Species Act Listing
Southern and Midwestern Turtle Species Affected by Commercial Harvest (in AR, FL, GA, IA, KY, LA, MO, OH, OK, SC, TN, and TX): species in red = depleted to the point they may warrant federal Endangered Species Act listing Common snapping turtle (Chelydra serpentina) – AR, GA, IA, KY, MO, OH, OK, SC, TX Florida common snapping turtle (Chelydra serpentina osceola) - FL Southern painted turtle (Chrysemys dorsalis) – AR Western painted turtle (Chrysemys picta) – IA, MO, OH, OK Spotted turtle (Clemmys gutatta) - FL, GA, OH Florida chicken turtle (Deirochelys reticularia chrysea) – FL Western chicken turtle (Deirochelys reticularia miaria) – AR, FL, GA, KY, MO, OK, TN, TX Barbour’s map turtle (Graptemys barbouri) - FL, GA Cagle’s map turtle (Graptemys caglei) - TX Escambia map turtle (Graptemys ernsti) – FL Common map turtle (Graptemys geographica) – AR, GA, OH, OK Ouachita map turtle (Graptemys ouachitensis) – AR, GA, OH, OK, TX Sabine map turtle (Graptemys ouachitensis sabinensis) – TX False map turtle (Graptemys pseudogeographica) – MO, OK, TX Mississippi map turtle (Graptemys pseuogeographica kohnii) – AR, TX Alabama map turtle (Graptemys pulchra) – GA Texas map turtle (Graptemys versa) - TX Striped mud turtle (Kinosternon baurii) – FL, GA, SC Yellow mud turtle (Kinosternon flavescens) – OK, TX Common mud turtle (Kinosternon subrubrum) – AR, FL, GA, OK, TX Alligator snapping turtle (Macrochelys temminckii) – AR, FL, GA, LA, MO, TX Diamond-back terrapin (Malaclemys terrapin) – FL, GA, LA, SC, TX River cooter (Pseudemys concinna) – AR, FL, -

Genus: Sternotherus (Musk Turtles) - Darrell Senneke Copyright © 2003 World Chelonian Trust
Genus: Sternotherus (Musk Turtles) - Darrell Senneke Copyright © 2003 World Chelonian Trust. All rights reserved Sternotherus carinatus - Razor-backed Musk Turtle Sternotherus depressus - Flattened Musk Turtle Sternotherus minor - Loggerhead Musk Turtle Sternotherus minor peltifer - Stripe-neck Musk Turtle Sternotherus odoratus - Common Musk Turtle This care sheet is intended only to cover the general care of these species. Further research to best develop a maintenance plan for whichever species you are caring for is essential.. Many taxonomists often combine genus Sternotherus with Kinosternon as sub- genera of the Family Kinosternidae. While these animals share many of the same habitats, features and care requirements, for the purpose of this care sheet they will be treated as a full Genus. Musk turtles can be found from the Canadian Southern border to Florida and West to the Rocky Mountains. These species are more carnivorous than most turtles with a natural diet that relies heavily on fish, snails, crustaceans and insects. While the Razor-back Musk turtle can attain a size of 15 cm. (6 inches), the much more commonly seen Stinkpot only attains 8 - 10 cm (3 - 4 inches) maximum. Present knowledge and technology make Musk turtles easily maintained animals as long as a person is willing to provide some basic requirements. Thanks to the success that breeders are having with these species it is now possible to purchase many of these species as hatchlings from captive born stock. Some of the species are threatened or endangered in nature, do not remove these animals from the wild. HOUSING MUSK TURTLES INDOORS - The most useful form of indoor accommodation for Sternotherus consists of an aquarium. -

Proposed Amendment to 21CFR124021
Richard Fife 8195 S. Valley Vista Drive Hereford, AZ 85615 December 07, 2015 Division of Dockets Management Food and Drug Administration 5630 Fishers Lane, rm. 1061 Rockville, MD 20852 Reference: Docket Number FDA-2013-S-0610 Proposed Amendment to Code of Federal Regulations Title 21, Volume 8 Revised as of April 1, 2015 21CFR Sec.1240.62 Dear Dr. Stephen Ostroff, M.D., Acting Commissioner: Per discussion with the Division of Dockets Management staff on November 10, 2015 Environmental and Economic impact statements are not required for petitions submitted under 21CFR Sec.1240.62 CITIZEN PETITION December 07, 2015 ACTION REQUESTED: I propose an amendment to 21CFR Sec.1240.62 (see exhibit 1) as allowed by Section (d) Petitions as follows: Amend section (c) Exceptions. The provisions of this section are not applicable to: By adding the following two (2) exceptions: (5) The sale, holding for sale, and distribution of live turtles and viable turtle eggs, which are sold for a retail value of $75 or more (not to include any additional turtle related apparatuses, supplies, cages, food, or other turtle related paraphernalia). This dollar amount should be reviewed every 5 years or more often, as deemed necessary by the department in order to make adjustments for inflation using the US Department of Labor, Bureau of labor Statistics, Consumer Price Index. (6) The sale, holding for sale, and distribution of live turtles and viable turtle eggs, which are listed by the International Union for Conservation of Nature and Natural Resources (IUCN) Red List as Extinct In Wild, Critically Endangered, Endangered, or Vulnerable (IUCN threatened categorizes). -

Heinrich and Walsh (2019)
THE BIG TURTLE YEAR LOOKING FOR WILD TURTLES IN WILD PLACES By George L. Heinrich and Timothy J. Walsh e like looking for wild turtles in wild We both liked turtles as kids, but now, many places. From the time George was in years later, we understand the important ecological Welementary school catching Wood roles they play. Some turtle species serve as indica- Turtles in southwestern Connecticut and Tim tors of environmental health, while others are clas- found his first pebble-sized Striped Mud Turtle sified as keystone species (those that play a vital at age 10 in a south Florida stream, we have both ecological role in a given habitat), umbrella spe- marveled at being in nature and searching for these cies (those whose conservation benefits the larger fascinating reptiles. As children, little did we know ecological community), or flagship species (iconic that our time spent exploring our neighborhood symbols of habitat conservation efforts). Perhaps woods would lead to rewarding careers in wildlife one of the best examples of the ecological signifi- conservation. Now we are both officers with the cance of turtles is the Gopher Tortoise, an imper- Florida Turtle Conservation Trust, an organization iled species that occurs in a six-state range within working to conserve Florida’s rich turtle diversity. the Southeastern Coastal Plain of the United States. In 2017 we created the opportunity of a Appropriately, the Gopher Tortoise was the first lifetime with the Trust’s conservation education species we found on day one of The Big Turtle Year. initiative: The Big Turtle Year, an ambitious plan to travel across the United States and back again SoutheaSt Regional highlightS trying to find as many species as we could. -

Chelonian Advisory Group Regional Collection Plan 4Th Edition December 2015
Association of Zoos and Aquariums (AZA) Chelonian Advisory Group Regional Collection Plan 4th Edition December 2015 Editor Chelonian TAG Steering Committee 1 TABLE OF CONTENTS Introduction Mission ...................................................................................................................................... 3 Steering Committee Structure ........................................................................................................... 3 Officers, Steering Committee Members, and Advisors ..................................................................... 4 Taxonomic Scope ............................................................................................................................. 6 Space Analysis Space .......................................................................................................................................... 6 Survey ........................................................................................................................................ 6 Current and Potential Holding Table Results ............................................................................. 8 Species Selection Process Process ..................................................................................................................................... 11 Decision Tree ........................................................................................................................... 13 Decision Tree Results ............................................................................................................. -

Turtles of the World, 2010 Update: Annotated Checklist of Taxonomy, Synonymy, Distribution, and Conservation Status
Conservation Biology of Freshwater Turtles and Tortoises: A Compilation ProjectTurtles of the IUCN/SSC of the World Tortoise – 2010and Freshwater Checklist Turtle Specialist Group 000.85 A.G.J. Rhodin, P.C.H. Pritchard, P.P. van Dijk, R.A. Saumure, K.A. Buhlmann, J.B. Iverson, and R.A. Mittermeier, Eds. Chelonian Research Monographs (ISSN 1088-7105) No. 5, doi:10.3854/crm.5.000.checklist.v3.2010 © 2010 by Chelonian Research Foundation • Published 14 December 2010 Turtles of the World, 2010 Update: Annotated Checklist of Taxonomy, Synonymy, Distribution, and Conservation Status TUR T LE TAXONOMY WORKING GROUP * *Authorship of this article is by this working group of the IUCN/SSC Tortoise and Freshwater Turtle Specialist Group, which for the purposes of this document consisted of the following contributors: ANDERS G.J. RHODIN 1, PE T ER PAUL VAN DI J K 2, JOHN B. IVERSON 3, AND H. BRADLEY SHAFFER 4 1Chair, IUCN/SSC Tortoise and Freshwater Turtle Specialist Group, Chelonian Research Foundation, 168 Goodrich St., Lunenburg, Massachusetts 01462 USA [[email protected]]; 2Deputy Chair, IUCN/SSC Tortoise and Freshwater Turtle Specialist Group, Conservation International, 2011 Crystal Drive, Suite 500, Arlington, Virginia 22202 USA [[email protected]]; 3Department of Biology, Earlham College, Richmond, Indiana 47374 USA [[email protected]]; 4Department of Evolution and Ecology, University of California, Davis, California 95616 USA [[email protected]] AB S T RAC T . – This is our fourth annual compilation of an annotated checklist of all recognized and named taxa of the world’s modern chelonian fauna, documenting recent changes and controversies in nomenclature, and including all primary synonyms, updated from our previous three checklists (Turtle Taxonomy Working Group [2007b, 2009], Rhodin et al. -

Sternotherus Minor (Agassiz) Loggerhead Musk Turtle 1
195.1 REPTILIA: TESTUDINES: KINOSTERNIDAE STERNOTHERUS MINOR Catalogue of American Amphibians' and Reptiles. River system in north Florida. Sternotherus m. peltifer occurs throughout the Pearl River drainage of eastern Louisiana and IVERSON,JOHN B. 1977. Sternotherus minor. Mississippi and the Mobile Bay drainage in Georgia, Tennessee, Alabama, and Mississippi. It also occurs in portions of the Tennessee River system in Tennessee, Alabama, and Virginia. Sternotherus minor (Agassiz) It is apparently absent from the Pascagoula River drainage Loggerhead musk turtle in Mississippi and Alabama. It occurs at least to 1500' above mean sea level (Johnson, 1958). Intergrade populations are found Goniochelys minor Agassiz, 1857:424. Type-localities, "neigh• in the Gulf drainages of the Perdido River to the Choctawhatchee borhood of Mobile [Mobile County, Alabama]," "Columbus River (Iverson, 1977). Sternotherus minor is not found at New [Muscogee County], Georgia" and "New Orleans [New Orleans, Louisiana, one of the original type localities. Orleans Parish, Louisiana]"; restricted to Columbus, Georgia, by Schmidt (1953). Syntypes: Alabama, Mus. Compo • FOSSILRECORD. There is a single Pleistocene record from Zool. 1570, subadult male, collected by Dr. Nott. Georgia, Vero, Indian River County, Florida (Weigel, 1962). Mus. Compo Zool. 1571 (2 specimens), adult female and • PERTINENT LITERATURE. General accounts of the biol• male, collected by Dr. Genner. Louisiana, U. S. Nat. Mus. ogy are given in Carr (1940, 1952), Tinkle (1958a), Ernst and 71111 (formerly Mus. Compo Zool. 1572), adult female col• Barbour (1972), and Conant (1975). Other important references lected by N. B. Benedict; Univ. Michigan Mus. Zool. 63520, are: taxonomy (Siebenrock, 1907; Stejneger, 1923; Tinkle and adult female collected by Dr. -

Central Florida Freshwater Turtle Research Group What Can You
What Can You Do? Population & Range Study Report Suspicious Activities Florida Peninsula Cooter Taking turtles from the Parks is illegal, and can (Pseudemys peninsularis) threaten population health. Report any suspected activity to Park officials. Conserve Natural Resources Central Florida Water is a precious resource, and is not unlimited. Freshwater Turtle Suwannee Cooter We must all do our part to conserve and preserve (Pseudemys concinna such resources so that they are available in the fu- Research Group suwanniensis) ture. Donate Time or Money Parks rely on external funding not only to expand their programs, but to continue those already in Florida Redbelly Cooter place. Research done by many groups is often paid (Pseudemys nelsoni) for by the researchers involved. Even small dona- tions of time or money add up! Educate Yourself, Speak Out, & Get Creative! Common Musk Turtle Understanding the basics of good science can help or Stinkpot (Sternotherus you become a better informed citizen. Share what odoratus) you know with others, and get together with friends and neighbors to find new ways to pitch in! Loggerhead Musk Turtle (Sternotherus minor minor) Wekiwa Springs State Park Florida Softshell Turtle (Apalone ferox) Rock Springs Run State Preserve Blue Springs State Park De Leon Springs State Park Eric Munscher Florida Snapping Turtle Principle Investigator & Herpetologist Manatee Springs State Park (Chelydra serpentina osceola) Email: [email protected] Organization Email: Fanning Springs State Park [email protected] Peacock Springs State Park Florida Chicken Turtle Website: (Deirochelys reticularia www.tg-fx.com/munscher_html/index.html chrysea) Facebook Group: Central Florida Freshwater Turtle Study Who we are In 2010, an additional study began in Wekiwa Springs to determine the natural range of Logger- The Central Florida Freshwater Turtle Study was be- head Musk Turtles and Stinkpots along the run of gun in 1999 by Dr. -

Japan's Trade of Live Freshwater Turtles and Tortoises As Pets
Japan’s Trade in Live Freshwater Turtles and Tortoises as Pets EXECUTIVE SUMMARY Almost 42%(128) of the world’s 305 species of turtles and tortoises are threatened with extinction (Baillie, Hilton-Taylor, Stuart, 2004). This endangerment comes both from loss of habitat and from direct use by humans. Across the world, people use turtles and tortoises in several ways, such as for food or for traditional medicine. In Japan, people buy turtles and tortoises as pets. Japan was the world’s largest importer of live tortoises Testudinidea spp., importing over 50% of the world’s total imports in 1996, for example (Anon., 1999). The desire for the rarest and most exotic forms means that Japan’s market for live freshwater turtles and tortoises are a significant factor in the endangerment of these species. This report investigates the trade of live turtles and tortoises as pets in Japan, describes the domestic control system for this trade, and recommends actions to ensure this trade is legal and sustainable. This report is based on surveys of pet shops, carried out in March 2002, as well as review of trade data from Japan’s government agencies. The global trade in turtles and tortoises often has a large volume, relative to the size of the populations of these turtles and tortoises. Most turtles and tortoises in trade are regulated internationally by the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES). CITES has taken a special interest in freshwater turtles and tortoises, adopting a Resolution on conservation of and trade in tortoises and freshwater turtles (Resolution Conf. -
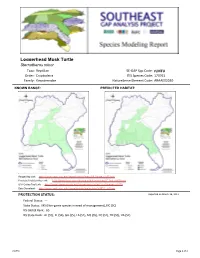
Loggerhead Musk Turtle
Loggerhead Musk Turtle Sternotherus minor Taxa: Reptilian SE-GAP Spp Code: rLMTU Order: Cryptodeira ITIS Species Code: 173761 Family: Kinosternidae NatureServe Element Code: ARAAE02030 KNOWN RANGE: PREDICTED HABITAT: P:\Proj1\SEGap P:\Proj1\SEGap Range Map Link: http://www.basic.ncsu.edu/segap/datazip/maps/SE_Range_rLMTU.pdf Predicted Habitat Map Link: http://www.basic.ncsu.edu/segap/datazip/maps/SE_Dist_rLMTU.pdf GAP Online Tool Link: http://www.gapserve.ncsu.edu/segap/segap/index2.php?species=rLMTU Data Download: http://www.basic.ncsu.edu/segap/datazip/region/vert/rLMTU_se00.zip PROTECTION STATUS: Reported on March 14, 2011 Federal Status: --- State Status: MS (Non-game species in need of management), NC (SC) NS Global Rank: G5 NS State Rank: AL (S5), FL (S4), GA (S5), LA (S1), MS (S5), NC (S1), TN (S5), VA (S2) rLMTU Page 1 of 4 SUMMARY OF PREDICTED HABITAT BY MANAGMENT AND GAP PROTECTION STATUS: US FWS US Forest Service Tenn. Valley Author. US DOD/ACOE ha % ha % ha % ha % Status 1 6,661.2 < 1 149.1 < 1 0.0 0 0.0 0 Status 2 5,674.3 < 1 4,034.7 < 1 0.0 0 0.0 0 Status 3 0.0 0 35,877.6 2 3,539.8 < 1 21,048.9 1 Status 4 1.0 < 1 0.0 0 0.0 0 0.0 0 Total 12,336.5 < 1 40,061.4 2 3,539.8 < 1 21,048.9 1 US Dept. of Energy US Nat. Park Service NOAA Other Federal Lands ha % ha % ha % ha % Status 1 0.0 0 279.3 < 1 0.0 0 28.4 < 1 Status 2 0.0 0 146.3 < 1 19.6 < 1 0.0 0 Status 3 378.1 < 1 413.1 < 1 0.0 0 402.6 < 1 Status 4 0.0 0 0.0 0 0.0 0 0.0 0 Total 378.1 < 1 838.6 < 1 19.6 < 1 431.0 < 1 Native Am.