Status of the Species – Red Knot U.S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
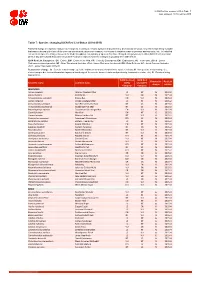
Table 7: Species Changing IUCN Red List Status (2014-2015)
IUCN Red List version 2015.4: Table 7 Last Updated: 19 November 2015 Table 7: Species changing IUCN Red List Status (2014-2015) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2014 (IUCN Red List version 2014.3) and 2015 (IUCN Red List version 2015-4) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2014) List (2015) change version Category Category MAMMALS Aonyx capensis African Clawless Otter LC NT N 2015-2 Ailurus fulgens Red Panda VU EN N 2015-4 -

Nordmann's Greenshank Population Analysis, at Pantai Cemara Jambi
Final Report Nordmann’s Greenshank Population Analysis, at Pantai Cemara Jambi Cipto Dwi Handono1, Ragil Siti Rihadini1, Iwan Febrianto1 and Ahmad Zulfikar Abdullah1 1Yayasan Ekologi Satwa Alam Liar Indonesia (Yayasan EKSAI/EKSAI Foundation) Surabaya, Indonesia Background Many shorebirds species have declined along East Asian-Australasian Flyway which support the highest diversity of shorebirds in the world, including the globally endangered species, Nordmann’s Greenshank. Nordmann’s Greenshank listed as endangered in the IUCN Red list of Threatened Species because of its small and declining population (BirdLife International, 2016). It’s one of the world’s most threatened shorebirds, is confined to the East Asian–Australasian Flyway (Bamford et al. 2008, BirdLife International 2001, 2012). Its global population is estimated at 500–1,000, with an estimated 100 in Malaysia, 100–200 in Thailand, 100 in Myanmar, plus unknown but low numbers in NE India, Bangladesh and Sumatra (Wetlands International 2006). The population is suspected to be rapidly decreasing due to coastal wetland development throughout Asia for industry, infrastructure and aquaculture, and the degradation of its breeding habitat in Russia by grazing Reindeer Rangifer tarandus (BirdLife International 2012). Mostly Nordmann’s Greenshanks have been recorded in very small numbers throughout Southeast Asia, and there are few places where it has been reported regularly. In Myanmar, for example, it was rediscovered after a gap of almost 129 years. The total count recorded by the Asian Waterbird Census (AWC) in 2006 for Myanmar was 28 birds with 14 being the largest number at a single locality (Naing 2007). In 2011–2012, Nordmann’s Greenshank was found three times in Sumatera Utara province, N Sumatra. -

Age-Related Differences in Ruddy Turnstone Foraging and Aggressive Behavior
AGE-RELATED DIFFERENCES IN RUDDY TURNSTONE FORAGING AND AGGRESSIVE BEHAVIOR SARAH GROVES ABSTRACT.--Theforaging behavior of fall migrant Ruddy Turnstoneswas studiedon the Mas- sachusettscoast on 2 different substrates,barnacle-covered rocks and sand and weed-litteredflats. Foragingrates differedsignificantly between the 2 substrates.On eachsubstrate the foragingand successrates of adults and juveniles differed significantly while the frequenciesof successwere similarfor both age-classes.The observeddifferences in foragingrates of adultsand juvenilesmay be due to the degreeof refinementof foragingtechniques. Experience in searchingfor and handling prey may be a primary factor accountingfor thesedifferences, and foragingperformance probably improves with age and experience.Alternatively, the differencesmay be due to the presenceof inefficient juveniles that do not survive to adulthood. Both adultsand juveniles in the tall-depressedposture were dominant in aggressiveinteractions much morefrequently than birds in the tall-levelposture. In mixedflocks of foragingadult and juvenile turnstones,the four possibletypes of aggressiveinteractions occurred nonrandomly. Adult over juvenile interactionsoccurred more frequently than expected,and juvenile over adult interac- tions were never seen.A tentative explanationof this phenomenonmay be that juveniles misinter- pret or respondambivalently to messagesconveyed behaviorally by adultsand thusbecome espe- cially vulnerableto aggressionby adults.The transiencyof migrantsmade it unfeasibleto evaluate -

No General Shift in Spring Migration Phenology by Eastern North American Birds Since 1970
bioRxiv preprint doi: https://doi.org/10.1101/2021.05.25.445655; this version posted May 26, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. No general shift in spring migration phenology by eastern North American birds since 1970 André Desrochers Département des sciences du bois et de la forêt, Université Laval, Québec, Canada Andra Florea Observatoire d’oiseaux de Tadoussac, Québec, Canada Pierre-Alexandre Dumas Observatoire d’oiseaux de Tadoussac, Québec, Canada, and Département des sci- ences du bois et de la forêt, Université Laval, Québec, Canada We studied the phenology of spring bird migration from eBird and ÉPOQ checklist programs South of 49°N in the province of Quebec, Canada, between 1970 and 2020. 152 species were grouped into Arctic, long-distance, and short-distance migrants. Among those species, 75 sig- nificantly changed their migration dates, after accounting for temporal variability in observation effort, species abundance, and latitude. But in contrast to most studies on the subject, we found no general advance in spring migration dates, with 36 species advancing and 39 species delaying their migration. Several early-migrant species associated to open water advanced their spring mi- gration, possibly due to decreasing early-spring ice cover in the Great Lakes and the St-Lawrence river since 1970. Arctic breeders and short-distance migrants advanced their first arrival dates more than long-distance migrants not breeding in the arctic. -

Population Analysis and Community Workshop for Far Eastern Curlew Conservation Action in Pantai Cemara, Desa Sungai Cemara – Jambi
POPULATION ANALYSIS AND COMMUNITY WORKSHOP FOR FAR EASTERN CURLEW CONSERVATION ACTION IN PANTAI CEMARA, DESA SUNGAI CEMARA – JAMBI Final Report Small Grant Fund of the EAAFP Far Eastern Curlew Task Force Iwan Febrianto, Cipto Dwi Handono & Ragil S. Rihadini Jambi, Indonesia 2019 The aim of this project are to Identify the condition of Far Eastern Curlew Population and the remaining potential sites for Far Eastern Curlew stopover in Sumatera, Indonesia and protect the remaining stopover sites for Far Eastern Curlew by educating the government, local people and community around the sites as the effort of reducing the threat of habitat degradation, habitat loss and human disturbance at stopover area. INTRODUCTION The Far Eastern Curlew (Numenius madagascariencis) is the largest shorebird in the world and is endemic to East Asian – Australian Flyway. It is one of the Endangered migratory shorebird with estimated global population at 38.000 individual, although a more recent update now estimates the population at 32.000 (Wetland International, 2015 in BirdLife International, 2017). An analysis of monitoring data collected from around Australia and New Zealand (Studds et al. in prep. In BirdLife International, 2017) suggests that the species has declined much more rapidly than was previously thought; with an annual rate of decline of 0.058 equating to a loss of 81.7% over three generations. Habitat loss occuring as a result of development is the most significant threat currently affecting migratory shorebird along the EAAF (Melville et al. 2016 in EAAFP 2017). Loss of habitat at critical stopover sites in the Yellow Sea is suspected to be the key threat to this species and given that it is restricted to East Asian - Australasian Flyway, the declines in the non-breeding are to be representative of the global population. -

<I>Actitis Hypoleucos</I>
Partial primary moult in first-spring/summer Common Sandpipers Actitis hypoleucos M. NICOLL 1 & P. KEMP 2 •c/o DundeeMuseum, Dundee, Tayside, UK 243 LochinverCrescent, Dundee, Tayside, UK Citation: Nicoll, M. & Kemp, P. 1983. Partial primary moult in first-spring/summer Common Sandpipers Actitis hypoleucos. Wader Study Group Bull. 37: 37-38. This note is intended to draw the attention of wader catch- and the old inner feathersare often retained (Pearson 1974). ers to the needfor carefulexamination of the primariesof Similarly, in Zimbabwe, first-year Common Sandpipers CommonSandpipers Actiris hypoleucos,and other waders, replacethe outerfive to sevenprimaries between December for partial primarywing moult. This is thoughtto be a diag- andApril (Tree 1974). It thusseems normal for first-spring/ nosticfeature of wadersin their first spring and summer summerCommon Sandpipers wintering in eastand southern (Tree 1974). Africa to show a contrast between new outer and old inner While membersof the Tay Ringing Group were mist- primaries.There is no informationfor birdswintering further nettingin Angus,Scotland, during early May 1980,a Com- north.However, there may be differencesin moult strategy mon Sandpiperdied accidentally.This bird was examined betweenwintering areas,since 3 of 23 juvenile Common and measured, noted as an adult, and then stored frozen un- Sandpiperscaught during autumn in Morocco had well- til it was skinned,'sexed', andthe gut contentsremoved for advancedprimary moult (Pienkowski et al. 1976). These analysis.Only duringskinning did we noticethat the outer birdswere moultingnormally, and so may have completed primarieswere fresh and unworn in comparisonto the faded a full primary moult during their first winter (M.W. Pien- and abradedinner primaries.The moult on both wingswas kowski, pers.comm.). -

Common Caribbean Shorebirds: ID Guide
Common Caribbean Shorebirds: ID Guide Large Medium Small 14”-18” 35 - 46 cm 8.5”-12” 22 - 31 cm 6”- 8” 15 - 20 cm Large Shorebirds Medium Shorebirds Small Shorebirds Whimbrel 17.5” 44.5 cm Lesser Yellowlegs 9.5” 24 cm Wilson’s Plover 7.75” 19.5 cm Spotted Sandpiper 7.5” 19 cm American Oystercatcher 17.5” 44.5 cm Black-bellied Plover 11.5” 29 cm Sanderling 7.75” 19.5 cm Western Sandpiper 6.5” 16.5 cm Willet 15” 38 cm Short-billed Dowitcher 11” 28 cm White-rumped Sandpiper 6” 15 cm Greater Yellowlegs 14” 35.5 cm Ruddy Turnstone 9.5” 24 cm Semipalmated Sandpiper 6.25” 16 cm 6.25” 16 cm American Avocet* 18” 46 cm Red Knot 10.5” 26.5 cm Snowy Plover Least Sandpiper 6” 15 cm 14” 35.5 cm 8.5” 21.5 cm Semipalmated Plover Black-necked Stilt* Pectoral Sandpiper 7.25” 18.5 cm Killdeer* 10.5” 26.5 cm Piping Plover 7.25” 18.5 cm Stilt Sandpiper* 8.5” 21.5 cm Lesser Yellowlegs & Ruddy Turnstone: Brad Winn; Red Knot: Anthony Levesque; Pectoral Sandpiper & *not pictured Solitary Sandpiper* 8.5” 21.5 cm White-rumped Sandpiper: Nick Dorian; All other photos: Walker Golder Clues to help identify shorebirds Size & Shape Bill Length & Shape Foraging Behavior Size Length Sandpipers How big is it compared to other birds? Peeps (Semipalmated, Western, Least) Walk or run with the head down, picking and probing Spotted Sandpiper Short Medium As long Longer as head than head Bobs tail up and down when walking Plovers, Turnstone or standing Small Medium Large Sandpipers White-rumped Sandpiper Tail tips up while probing Yellowlegs Overall Body Shape Stilt Sandpiper Whimbrel, Oystercatcher, Probes mud like “oil derrick,” Willet, rear end tips up Dowitcher, Curvature Plovers Stilt, Avocet Run & stop, pick, hiccup, run & stop Elongate Compact Yellowlegs Specific Body Parts Stroll and pick Bill & leg color Straight Upturned Dowitchers Eye size Plovers = larger, sandpipers = smaller Tip slightly Probe mud with “sewing machine” Leg & neck length downcurved Downcurved bill, body stays horizontal . -

The Systematic Position of the Surfbird, Aphriza Virgata
THE SYSTEMATIC POSITION OF THE SURFBIRD, APHRIZA VIRGATA JOSEPH R. JEHL, JR. University of Michigan Museum of Zoology Ann Arbor, Michigan 48104 The taxonomic relationships of the Surfbird, ( 1884) elevated the tumstone-Surfbird unit Aphriza virgata, have long been one of the to family rank. But, although they stated (p. most controversial problems in shorebird clas- 126) that Aphrizu “agrees very closely” with sification. Although the species has been as- Arenaria, the only points of similarity men- signed to a monotypic family (Shufeldt 1888; tioned were “robust feet, without trace of web Ridgway 1919), most modern workers agree between toes, the well formed hind toe, and that it should be placed with the turnstones the strong claws; the toes with a lateral margin ( Arenaria spp. ) in the subfamily Arenariinae, forming a broad flat under surface.” These even though they have reached no consensuson differences are hardly sufficient to support the affinities of this subfamily. For example, familial differentiation, or even to suggest Lowe ( 1931), Peters ( 1934), Storer ( 1960), close generic relationship. and Wetmore (1965a) include the Arenariinae Coues (1884605) was uncertain about the in the Scolopacidae (sandpipers), whereas Surfbirds’ relationships. He called it “a re- Wetmore (1951) and the American Ornithol- markable isolated form, perhaps a plover and ogists ’ Union (1957) place it in the Charadri- connecting this family with the next [Haema- idae (plovers). The reasons for these diverg- topodidae] by close relationships with Strep- ent views have never been stated. However, it silas [Armaria], but with the hind toe as well seems that those assigning the Arenariinae to developed as usual in Sandpipers, and general the Charadriidae have relied heavily on their appearance rather sandpiper-like than plover- views of tumstone relationships, because schol- like. -

Purple Sandpiper
Maine 2015 Wildlife Action Plan Revision Report Date: January 13, 2016 Calidris maritima (Purple Sandpiper) Priority 1 Species of Greatest Conservation Need (SGCN) Class: Aves (Birds) Order: Charadriiformes (Plovers, Sandpipers, And Allies) Family: Scolopacidae (Curlews, Dowitchers, Godwits, Knots, Phalaropes, Sandpipers, Snipe, Yellowlegs, And Woodcock) General comments: Recent surveys suggest population undergoing steep population decline within 10 years. IFW surveys conducted in 2014 suggest population declined by 49% since 2004 (IFW unpublished data). Maine has high responsibility for wintering population, regional surveys suggest Maine may support over 1/3 of the Western Atlantic wintering population. USFWS Region 5 and Canadian Maritimes winter at least 90% of the Western Atlantic population. Species Conservation Range Maps for Purple Sandpiper: Town Map: Calidris maritima_Towns.pdf Subwatershed Map: Calidris maritima_HUC12.pdf SGCN Priority Ranking - Designation Criteria: Risk of Extirpation: NA State Special Concern or NMFS Species of Concern: NA Recent Significant Declines: Purple Sandpiper is currently undergoing steep population declines, which has already led to, or if unchecked is likely to lead to, local extinction and/or range contraction. Notes: Recent surveys suggest population undergoing steep population decline within 10 years. IFW surveys conducted in 2014 suggest population declined by 49% since 2004 (IFW unpublished data). Maine has high responsibility for wintering populat Regional Endemic: Calidris maritima's global geographic range is at least 90% contained within the area defined by USFWS Region 5, the Canadian Maritime Provinces, and southeastern Quebec (south of the St. Lawrence River). Notes: Recent surveys suggest population undergoing steep population decline within 10 years. IFW surveys conducted in 2014 suggest population declined by 49% since 2004 (IFW unpublished data). -

Birds of Chile a Photo Guide
© Copyright, Princeton University Press. No part of this book may be 88 distributed, posted, or reproduced in any form by digital or mechanical 89 means without prior written permission of the publisher. WALKING WATERBIRDS unmistakable, elegant wader; no similar species in Chile SHOREBIRDS For ID purposes there are 3 basic types of shorebirds: 6 ‘unmistakable’ species (avocet, stilt, oystercatchers, sheathbill; pp. 89–91); 13 plovers (mainly visual feeders with stop- start feeding actions; pp. 92–98); and 22 sandpipers (mainly tactile feeders, probing and pick- ing as they walk along; pp. 99–109). Most favor open habitats, typically near water. Different species readily associate together, which can help with ID—compare size, shape, and behavior of an unfamiliar species with other species you know (see below); voice can also be useful. 2 1 5 3 3 3 4 4 7 6 6 Andean Avocet Recurvirostra andina 45–48cm N Andes. Fairly common s. to Atacama (3700–4600m); rarely wanders to coast. Shallow saline lakes, At first glance, these shorebirds might seem impossible to ID, but it helps when different species as- adjacent bogs. Feeds by wading, sweeping its bill side to side in shallow water. Calls: ringing, slightly sociate together. The unmistakable White-backed Stilt left of center (1) is one reference point, and nasal wiek wiek…, and wehk. Ages/sexes similar, but female bill more strongly recurved. the large brown sandpiper with a decurved bill at far left is a Hudsonian Whimbrel (2), another reference for size. Thus, the 4 stocky, short-billed, standing shorebirds = Black-bellied Plovers (3). -

Birds of the East Texas Baptist University Campus with Birds Observed Off-Campus During BIOL3400 Field Course
Birds of the East Texas Baptist University Campus with birds observed off-campus during BIOL3400 Field course Photo Credit: Talton Cooper Species Descriptions and Photos by students of BIOL3400 Edited by Troy A. Ladine Photo Credit: Kenneth Anding Links to Tables, Figures, and Species accounts for birds observed during May-term course or winter bird counts. Figure 1. Location of Environmental Studies Area Table. 1. Number of species and number of days observing birds during the field course from 2005 to 2016 and annual statistics. Table 2. Compilation of species observed during May 2005 - 2016 on campus and off-campus. Table 3. Number of days, by year, species have been observed on the campus of ETBU. Table 4. Number of days, by year, species have been observed during the off-campus trips. Table 5. Number of days, by year, species have been observed during a winter count of birds on the Environmental Studies Area of ETBU. Table 6. Species observed from 1 September to 1 October 2009 on the Environmental Studies Area of ETBU. Alphabetical Listing of Birds with authors of accounts and photographers . A Acadian Flycatcher B Anhinga B Belted Kingfisher Alder Flycatcher Bald Eagle Travis W. Sammons American Bittern Shane Kelehan Bewick's Wren Lynlea Hansen Rusty Collier Black Phoebe American Coot Leslie Fletcher Black-throated Blue Warbler Jordan Bartlett Jovana Nieto Jacob Stone American Crow Baltimore Oriole Black Vulture Zane Gruznina Pete Fitzsimmons Jeremy Alexander Darius Roberts George Plumlee Blair Brown Rachel Hastie Janae Wineland Brent Lewis American Goldfinch Barn Swallow Keely Schlabs Kathleen Santanello Katy Gifford Black-and-white Warbler Matthew Armendarez Jordan Brewer Sheridan A. -

Biogeographical Profiles of Shorebird Migration in Midcontinental North America
U.S. Geological Survey Biological Resources Division Technical Report Series Information and Biological Science Reports ISSN 1081-292X Technology Reports ISSN 1081-2911 Papers published in this series record the significant find These reports are intended for the publication of book ings resulting from USGS/BRD-sponsored and cospon length-monographs; synthesis documents; compilations sored research programs. They may include extensive data of conference and workshop papers; important planning or theoretical analyses. These papers are the in-house coun and reference materials such as strategic plans, standard terpart to peer-reviewed journal articles, but with less strin operating procedures, protocols, handbooks, and manu gent restrictions on length, tables, or raw data, for example. als; and data compilations such as tables and bibliogra We encourage authors to publish their fmdings in the most phies. Papers in this series are held to the same peer-review appropriate journal possible. However, the Biological Sci and high quality standards as their journal counterparts. ence Reports represent an outlet in which BRD authors may publish papers that are difficult to publish elsewhere due to the formatting and length restrictions of journals. At the same time, papers in this series are held to the same peer-review and high quality standards as their journal counterparts. To purchase this report, contact the National Technical Information Service, 5285 Port Royal Road, Springfield, VA 22161 (call toll free 1-800-553-684 7), or the Defense Technical Infonnation Center, 8725 Kingman Rd., Suite 0944, Fort Belvoir, VA 22060-6218. Biogeographical files o Shorebird Migration · Midcontinental Biological Science USGS/BRD/BSR--2000-0003 December 1 By Susan K.