Tasmanian Bird Report 38
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Table 7: Species Changing IUCN Red List Status (2014-2015)
IUCN Red List version 2015.4: Table 7 Last Updated: 19 November 2015 Table 7: Species changing IUCN Red List Status (2014-2015) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2014 (IUCN Red List version 2014.3) and 2015 (IUCN Red List version 2015-4) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2014) List (2015) change version Category Category MAMMALS Aonyx capensis African Clawless Otter LC NT N 2015-2 Ailurus fulgens Red Panda VU EN N 2015-4 -

Nordmann's Greenshank Population Analysis, at Pantai Cemara Jambi
Final Report Nordmann’s Greenshank Population Analysis, at Pantai Cemara Jambi Cipto Dwi Handono1, Ragil Siti Rihadini1, Iwan Febrianto1 and Ahmad Zulfikar Abdullah1 1Yayasan Ekologi Satwa Alam Liar Indonesia (Yayasan EKSAI/EKSAI Foundation) Surabaya, Indonesia Background Many shorebirds species have declined along East Asian-Australasian Flyway which support the highest diversity of shorebirds in the world, including the globally endangered species, Nordmann’s Greenshank. Nordmann’s Greenshank listed as endangered in the IUCN Red list of Threatened Species because of its small and declining population (BirdLife International, 2016). It’s one of the world’s most threatened shorebirds, is confined to the East Asian–Australasian Flyway (Bamford et al. 2008, BirdLife International 2001, 2012). Its global population is estimated at 500–1,000, with an estimated 100 in Malaysia, 100–200 in Thailand, 100 in Myanmar, plus unknown but low numbers in NE India, Bangladesh and Sumatra (Wetlands International 2006). The population is suspected to be rapidly decreasing due to coastal wetland development throughout Asia for industry, infrastructure and aquaculture, and the degradation of its breeding habitat in Russia by grazing Reindeer Rangifer tarandus (BirdLife International 2012). Mostly Nordmann’s Greenshanks have been recorded in very small numbers throughout Southeast Asia, and there are few places where it has been reported regularly. In Myanmar, for example, it was rediscovered after a gap of almost 129 years. The total count recorded by the Asian Waterbird Census (AWC) in 2006 for Myanmar was 28 birds with 14 being the largest number at a single locality (Naing 2007). In 2011–2012, Nordmann’s Greenshank was found three times in Sumatera Utara province, N Sumatra. -

Governor Island MARINE RESERVE
VISITING RESERVES Governor Island MARINE RESERVE Governor Island Marine Reserve, with its spectacular underwater scenery, is recognised as one of the best temperate diving locations in Australia. The marine reserve includes Governor Island and all waters and other islands within a 400m diameter semi-circle from the eastern shoreline of Governor Island (refer map). The entire marine reserve is a fully protected ‘no-take’ area. Fishing and other extractive activities are prohibited. Yellow zoanthids adorn granite boulder walls in the marine reserve. These flower-like animals use their tentacles to catch tiny food particles drifting past in the current. Getting there Photo: Karen Gowlett-Holmes Governor Island lies just off Bicheno – a small fishing and resort town on Tasmania’s east coast. It is located Things to do about two and a half hours drive from either Hobart or The reserve is a popular diving location with Launceston. over 35 recognised dive sites, including: Governor Island is separated from the mainland by a The Hairy Wall – a granite cliff-face plunging to 35m, narrow stretch of water, approximately 50m wide, known with masses of sea whips as Waubs Gulch. For your safety please do not swim, The Castle – two massive granite boulders, sandwiched snorkel or dive in Waubs Gulch. It is subject to frequent together, with a swim-through lined with sea whips and boating traffic and strong currents and swells. The marine yellow zoanthids, and packed with schools of bullseyes, cardinalfish, banded morwong and rock lobster reserve is best accessed via commercial operators or LEGEND Golden Bommies – two 10m high pinnacles glowing with private boat. -

The Systematic Position of the Surfbird, Aphriza Virgata
THE SYSTEMATIC POSITION OF THE SURFBIRD, APHRIZA VIRGATA JOSEPH R. JEHL, JR. University of Michigan Museum of Zoology Ann Arbor, Michigan 48104 The taxonomic relationships of the Surfbird, ( 1884) elevated the tumstone-Surfbird unit Aphriza virgata, have long been one of the to family rank. But, although they stated (p. most controversial problems in shorebird clas- 126) that Aphrizu “agrees very closely” with sification. Although the species has been as- Arenaria, the only points of similarity men- signed to a monotypic family (Shufeldt 1888; tioned were “robust feet, without trace of web Ridgway 1919), most modern workers agree between toes, the well formed hind toe, and that it should be placed with the turnstones the strong claws; the toes with a lateral margin ( Arenaria spp. ) in the subfamily Arenariinae, forming a broad flat under surface.” These even though they have reached no consensuson differences are hardly sufficient to support the affinities of this subfamily. For example, familial differentiation, or even to suggest Lowe ( 1931), Peters ( 1934), Storer ( 1960), close generic relationship. and Wetmore (1965a) include the Arenariinae Coues (1884605) was uncertain about the in the Scolopacidae (sandpipers), whereas Surfbirds’ relationships. He called it “a re- Wetmore (1951) and the American Ornithol- markable isolated form, perhaps a plover and ogists ’ Union (1957) place it in the Charadri- connecting this family with the next [Haema- idae (plovers). The reasons for these diverg- topodidae] by close relationships with Strep- ent views have never been stated. However, it silas [Armaria], but with the hind toe as well seems that those assigning the Arenariinae to developed as usual in Sandpipers, and general the Charadriidae have relied heavily on their appearance rather sandpiper-like than plover- views of tumstone relationships, because schol- like. -

Alonnah-Sheepwash Track (Bruny Island) Weed Action Plan 2020 - 2030
Alonnah-Sheepwash Track (Bruny Island) Weed Action Plan 2020 - 2030 A funding initiative of the Bruny Island Destination Action Plan Leadership Group Locally supported by Acknowledgements 3 Abbreviations 3 1.0: Introduction 4 1.1: Plan Goal 4 1.2: Study Area 4 1.3: Priority Weeds 6 1.4: Relevant Plans and Legislation 9 1.5: Weed Action Principles 10 1.6: Weed Control Methods 10 1.7: Responsibility 10 1.8: Hygiene 11 1.9: Living Appendix 11 2.0: Site Maps 12 2.1: Zone 1 12 2.2: Zone 2 13 2.3: Zone 3 14 2.4: Zone 4 15 2.5: Zone 5 16 2.6: Zone 6 17 2.7: Zone 7 18 2.8: Zone 8 19 3.0: Weed Actions 20 3.1: Action Plan 20 4.0: Photo Point Monitoring Pro Forma 27 References 28 Appendix 1 – Stakeholders 29 Key Stakeholders 29 Other Stakeholders 30 Appendix 2 – Priority Weeds; Spread and Control 31 Appendix 3 – Native Plant List 34 Alonnah-Sheepwash Track Weed Action Plan 2020-2030 – DRAFT FOR CONSULTATION 2 Acknowledgements The Alonnah-Sheepwash Weed Action Plan is jointly funded by Pennicott Wilderness Tours, Kingborough Council and the Tasmanian Government, as an initiative of the Bruny Island Destination Action Plan (DAP). The DAP Leadership Group is coordinated by Destination Southern Tasmania. The funding for the plan is being locally auspiced by the Bruny Island Boat Club. Communications and networking associated with the plan is being undertaken by members of the Bruny Island Residents and Ratepayers Network (‘Bruny Network’). Cassandra Strain is the principal author of this strategy. -

Pages 345–366850.31 KB
Conservation Science W. Aust. 8 (3) : 345–366 (2013) Wader numbers and distribution on Eighty Mile Beach, north-west Australia: baseline counts for the period 1981–2003 CLIVE MINTON 1, MICHAEL CONNOR 2, DAVID PRICE 3, ROSALIND JESSOP 4, PETER COLLINS 5, HUMPHREY SITTERS 6, CHRIS HASSELL 7, GRANT PEARSON 8, DANNY ROGERS 9 1 165 Dalgetty Road Beaumaris, Victoria 3193 2 19 Pamela Grove Lower Templestowe, Victoria 3107 [email protected] 3 8 Scattor View Bridford, Exeter, Devon EX6 7JF, UK 4 Phillip Island Nature Park, PO Box 97 Cowes, Victoria 3922 5 214 Doveton Crescent Soldiers Hill, Ballarat, Victoria 3350 6 Higher Wyndcliffe Barline, Beer, Seaton, Devon EX12 3LP, UK 7 PO Box 3089 Broome, Western Australia 6725 8 Western Australian Department of Parks and Wildlife, PO Box 51 Wanneroo, Western Australia 6065 9 340 Ninks Road St Andrews, Victoria 3761 ABSTRACT This paper analyses ground counts and aerial surveys of high-tide wader roosts conducted over the 23-year period from 1981 to 2003, at Eighty Mile Beach, north-west Australia. It provides a baseline data set with which later count data can be compared. Over the study period, Eighty Mile Beach held a maximum of around 470,000 waders in any given year. This represented around 20% of the total number of migratory waders visiting Australia each year and around 6% of the total East Asian – Australasian Flyway migratory wader population. The most numerous species were great knot (169,000), bar-tailed godwit (110,000), greater sand plover (65,000) and oriental plover (58,000). -

YELLOW THROAT the Newsletter of Birdlife Tasmania: a Branch of Birdlife Australia Number 110, Winter 2020
YELLOW THROAT The newsletter of BirdLife Tasmania: a branch of BirdLife Australia Number 110, Winter 2020 Welcome to all our new readers (supporters and new Contents members) to the Winter edition of Yellow Throat. Masked Lapwing—Is it breeding earlier this year…..2 Normally we would be letting you know when the Concern for Tasmania’s woodland birds………………...3 next BirdLife Tasmania General Meeting will be held and who will be speaking. Alas, we are still under More of the same—windfarms gaining approval through archaic assessment……………………………………..4 COVID-19 restrictions for now, and are unsure when the next meeting will take place, but it will not be be- Birdata is easy to use and helps our birdlife!...............7 fore September. Beneath the radar…………………………………………………….8 We will continue to provide updates in the e-bulletin What Happens to Out-of-Range Records in on the resumption of meetings, and also, of course, Birdata?.................................................................... .10 outings. At this stage, outings will hopefully resume in Any more oddities?....................................................11 August. In the meantime, enjoy the many interesting Bird - safe architecture…………………………………………. .12 articles we have in this issue of Yellow Throat; we South-east Tasmanian KBA report indicates climate - hope you are making the most of the birds in your related concern for some species………………………… ..14 local area. Birding in backyards initiative………………………………. ..17 In this issue of Yellow Throat, two programs are out- lined that allow the community to participate in bird Is your cat a killer?......................................................18 surveys. General Birdata surveys and Birds in Back- Cat management in Tasmania………………… .19 yards surveys are two different ways that people who Letter from the Raptor Refuge………………………………..20 love birds can record what they see. -

How the Macquarie Island Parakeet Became Extinct
42 NEW ZEALAND JOURNAL OF ECOLOGY, VOL. 2. 1979 HOW THE MACQUARIE ISLAND PARAKEET BECAME EXTINCT R.H.TAYLOR Ecology Division, DSIR. Private Bag, Nelson SUMMARY: For 70 years following the discovery of Macquarie Island in 1810 the endemic parakeet Cyanoramphus novaezelandiae erythrotis remained plentiful, despite the introduction of cats (Felis catus) and other predators. The crucial factor in the bird's rapid disappearance between 1881 and 1890 appears to have been the successful liberation of rabbits (Oryctolagus cuniculus) in 1879. This led to great increases of feral cats and introduced wekas (Gallirallus australis) and presumably to greatly intensified predation on parakeets. INTRODUCTION their oil began in the 1870s and continued until 1918 When subantarctic Macquarie Island (54∞ 30'S, (Cumpston, 1968). As early as 1815 it was reported that there were 159∞E) was discovered in 1810 it had an endemic parakeet, Cyanoramphus novaezelandiae erythrotis. "innumerable wild dogs" on the island, which were causing great destruction to the bird life. Belling- Little has been recorded of the bird's habits but it hausen in 1820 found both feral dogs and cats was widespread and abundant, nesting in and under established. There is no record of feral dogs after tussocks on the tree-less island (Cumpston, 1968). 1820, and presumably they died out from starvation These parakeets were particularly common around when sealing became sporadic and when easily the shore, where they fed on invertebrates from obtainable winter food in the form of surface-nesting heaps of seaweed. For 70 years after discovery, albatrosses had been destroyed. However, most parakeets remained plentiful on the island, but after sealing parties continued to bring trained dogs to 1880 they disappeared very rapidly and were extinct the island to hunt birds for food (Cumpston, 1968). -

3966 Tour Op 4Col
The Tasmanian Advantage natural and cultural features of Tasmania a resource manual aimed at developing knowledge and interpretive skills specific to Tasmania Contents 1 INTRODUCTION The aim of the manual Notesheets & how to use them Interpretation tips & useful references Minimal impact tourism 2 TASMANIA IN BRIEF Location Size Climate Population National parks Tasmania’s Wilderness World Heritage Area (WHA) Marine reserves Regional Forest Agreement (RFA) 4 INTERPRETATION AND TIPS Background What is interpretation? What is the aim of your operation? Principles of interpretation Planning to interpret Conducting your tour Research your content Manage the potential risks Evaluate your tour Commercial operators information 5 NATURAL ADVANTAGE Antarctic connection Geodiversity Marine environment Plant communities Threatened fauna species Mammals Birds Reptiles Freshwater fishes Invertebrates Fire Threats 6 HERITAGE Tasmanian Aboriginal heritage European history Convicts Whaling Pining Mining Coastal fishing Inland fishing History of the parks service History of forestry History of hydro electric power Gordon below Franklin dam controversy 6 WHAT AND WHERE: EAST & NORTHEAST National parks Reserved areas Great short walks Tasmanian trail Snippets of history What’s in a name? 7 WHAT AND WHERE: SOUTH & CENTRAL PLATEAU 8 WHAT AND WHERE: WEST & NORTHWEST 9 REFERENCES Useful references List of notesheets 10 NOTESHEETS: FAUNA Wildlife, Living with wildlife, Caring for nature, Threatened species, Threats 11 NOTESHEETS: PARKS & PLACES Parks & places, -

Post-Breeding Stopover Sites of Waders in the Estuaries of the Khairusovo, Belogolovaya and Moroshechnaya Rivers, Western Kamchatka Peninsula, Russia, 2010–2012
Post-breeding stopover sites of waders in the estuaries of the Khairusovo, Belogolovaya and Moroshechnaya rivers, western Kamchatka Peninsula, Russia, 2010–2012 Dmitry S. Dorofeev1 & Fedor V. Kazansky2 1 All-Russian Research Institute for Nature Protection (ARRINP), Znamenskoe-Sadki, Moscow, 117628 Russia [email protected] 2 Kronotsky State Biosphere Reserve, Ryabikova St. 48, Elizovo, Kamchatskiy Kray, 68400 Russia. [email protected] Dorofeev, D.S. & Kazansky, F.V. 2013. Post-breeding stopover sites of waders in the estuaries of the Khairusovo, Belogolovaya and Moroshechnaya rivers, western Kamchatka Peninsula, Russia, 2010–2012. Wader Study Group Bull. 120(2): 119–123. Keywords: East Asian–Australasian Flyway, Kamchatka, waders, stopover site, resightings During the northern summer and autumn seasons of 2010–2012 we collected data on the numbers of waders that stop on the estuaries of the rivers Khairusovo, Belogolovaya and Moroshechnaya on the west-central coast of the Kamchatka Peninsula, Russia. Among known wader stopovers on the west coast of Kamchatka, this is the area that supports the largest numbers. We found that the most abundant species were Great Knot Calidris tenuirostris, Bar-tailed Godwit Limosa lapponica, Black-tailed Godwit L. limosa and Red-necked Stint C. ruficollis. Two globally-threatened species were also recorded: Eastern Curlew Numenius madagascarensis and Spoon-billed Sandpiper Eurynorhynchus pygmeus. At least 35 Great Knots colour-marked in NW Australia, one from South Australia and two from China were recorded in the area. We also observed several colour- marked Bar-tailed Godwits, Red-necked Stints and a Ruddy Turnstone Arenaria interpres marked in different areas of Australia and in China. -

EPBC Act Referral Is Complete, Current and Correct
Submission #2895 - Robbins Island Renewable Energy Park, Robbins Island, Tasmania Title of Proposal - Robbins Island Renewable Energy Park, Robbins Island, Tasmania Section 1 - Summary of your proposed action Provide a summary of your proposed action, including any consultations undertaken. 1.1 Project Industry Type Energy Generation and Supply (renewable) 1.2 Provide a detailed description of the proposed action, including all proposed activities. Please see letter for detailed description of the proposed action, attached at 1.4. 1.3 What is the extent and location of your proposed action? Use the polygon tool on the map below to mark the location of your proposed action. Area Point Latitude Longitude Robbins Island 1 -40.674899476105 145.00381304524 Robbins Island 2 -40.678232305635 145.00655962727 Robbins Island 3 -40.68156496855 145.01397539038 Robbins Island 4 -40.684689176792 145.02660966353 Robbins Island 5 -40.68427259873 145.03155351957 Robbins Island 6 -40.68427259873 145.03484940962 Robbins Island 7 -40.682606387569 145.03924395764 Robbins Island 8 -40.682189796489 145.0417158647 Robbins Island 9 -40.680315231537 145.04528642972 Robbins Island 10 -40.680106929163 145.04858231978 Robbins Island 11 -40.680106929163 145.04968095678 Robbins Island 12 -40.682189796489 145.04775835774 Robbins Island 13 -40.68156496855 145.04611041271 Robbins Island 14 -40.683231205745 145.04446244673 Robbins Island 15 -40.684689176792 145.04501177571 Robbins Island 16 -40.685522261546 145.04720902876 Robbins Island 17 -40.686147084167 145.0447371217 -
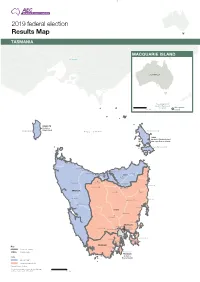
Tasmania Results
2019 federal election Results Map TASMANIA MACQUARIE ISLAND VICTORIA AUSTRALIA Macquarie Island is part of the Division of Franklin. Macquarie 0 1000 km Island BRADDON (includes King Island) King Island BASS STRAIT Flinders Island BASS (includes Flinders Island and Cape Barren Island) Cape Barren Island Smithton Wynyard Bridport Burnie George Town Ulverstone Branxholm Devonport BASS Lilydale Shefeld Waratah Launceston Scamander Mole Creek Evandale BRADDON Cressy Fingal Zeehan Bicheno Campbell Town Queenstown Swansea LYONS Oatlands Bothwell Orford FRANKLIN Bridgewater Maydena Richmond New Norfolk Sorell Eaglehawk Neck Franklin Cygnet Nubeena FRANKLIN Key Dover Division boundary LYONS Division name Bruny Island FRANKLIN (includes Party Bruny Island) Liberal Party* Australian Labor Party * Liberal/National Coalition The electoral boundaries represented on this map are those in place at the 2019 election. 0 50 km 2019 federal election Results Map TASMANIA Hobart Urban Tea Tree Bridgewater River Austins Ferry FRANKLIN Claremont Derwent Chigwell Dowsing Point Risdon Vale Berriedale Glenlusk Rosetta Montrose Derwent Park Lutana Glenorchy Collinsvale FRANKLIN Moonah West Moonah New Town Lenah North Valley Hobart Bellerive West Hobart Hobart Wellington Park CLARK Battery Point South Hobart Dynnyrne Sandy Bay Mount Nelson Ridgeway Fern Tree Taroona Neika Bonnet Longley Hill Leslie Vale Kingston Key Division boundary CLARK Division name Party Independent The electoral boundaries represented on this map are those in place at the 2019 election. 0 2 km.