Arterial Identity of Endothelial Cells Is Controlled by Local Cues
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Glossary of Key Terms and Concepts - Chapter 8
Glossary of Key Terms and Concepts - Chapter 8 Angioblasts - These "vessel-forming cells" may arise from any kind of mesoderm except prechordal plate mesoderm. Angioblastic cords - Angiocysts coalesce to form short blind-ended angioblastic cords. Angioblastic plexuses - Angioblastic cords coalesce to form complex interconnected vascular networks or plexuses. Angiocysts - These vesicles are formed by angioblasts during the process of vasculogenesis. Angiogenesis - This is the mechanism whereby preexisting vessels lengthen or branch by sprouting. Aortic arches - These vessels have been modified in humans to form the great vessels of the thorax (also see Ch. 7). Axis arteries - These central arteries of the limbs are derived from the 7th intersegmental arteries (upper limb) and 5th lumbar intersegmental arteries (lower limb). Blood islands - Blood islands are cysts of angioblasts containing hemoblasts. These coalesce to form blood vessels in the yolk sac and also form the coronary vasculature. Branchial arches - These are the gill bars of fish. Homologous structures of humans are more appropriately named "pharyngeal" arches. Cardinal system of veins - These veins drain the head and neck and body wall and extremities of the embryo. Anterior cardinals drain the head and neck and the trunk and lower extremities are drained by paired posterior cardinals. The posterior cardinal veins are replaced by subcardinal and supracardinal veins during the second month. Coronary vessels - These vessels of the heart form from epicardium as subepicardial plexuses fuse with sprouts of the aorta and coronary sinus to form the coronary arteries and coronary veins respectively. Endothelial cells - These cells arise from angioblasts to form the initial vascular network. -

Cardiovascular System Heart Development Cardiovascular System Heart Development
Cardiovascular System Heart Development Cardiovascular System Heart Development In human embryos, the heart begins to beat at approximately 22-23 days, with blood flow beginning in the 4th week. The heart is one of the earliest differentiating and functioning organs. • This emphasizes the critical nature of the heart in distributing blood through the vessels and the vital exchange of nutrients, oxygen, and wastes between the developing baby and the mother. • Therefore, the first system that completes its development in the embryo is called cardiovascular system. https://www.slideshare.net/DrSherifFahmy/intraembryonic-mesoderm-general-embryology Mesoderm is one of the three • Connective tissue primary germ layers that • Smooth and striated muscle • Cardiovascular System differentiates early in • Kidneys development that collectively • Spleen • Genital organs, ducts gives rise to all subsequent • Adrenal gland cortex tissues and organs. The cardiovascular system begins to develop in the third week of gestation. Blood islands develop in the newly formed mesoderm, and consist of (a) a central group of haemoblasts, the embryonic precursors of blood cells; (b) endothelial cells. Development of the heart and vascular system is often described together as the cardiovascular system. Development begins very early in mesoderm both within (embryonic) and outside (extra embryonic, vitelline, umblical and placental) the embryo. Vascular development occurs in many places. • Blood islands coalesce to form a vascular plexus. Preferential channels form arteries and veins. • Day 17 - Blood islands form first in the extra-embryonic mesoderm • Day 18 - Blood islands form next in the intra-embryonic mesoderm • Day 19 - Blood islands form in the cardiogenic mesoderm and coalesce to form a pair of endothelial heart tubes Development of a circulation • A circulation is established during the 4th week after the myocardium is differentiated. -

Development of the Vascular System in Five to Twenty-One
THE DEVELOPMENT OF THE VASCULAR SYSTEM IN FIVE TO TWtNTY-ONE SOMITE DOG EMBRYOS by ELDEN WILLIAM MARTIN B, S., Kansas State College of Agriculture and ADolied Science, 195>U A THESIS submitted in partial fulfillment of the requirements for the degree MASTER OF SCIENCE Department of Zoology KANSAS STATV: COLLEGE OF AGRICULTURE AND A PLIED SCIENCE 1958 LP TH Ooco/*>*Tv TABLE OF CONTENTS INTRO IXJ CTION AND LITERATURE REVIEW 1 MATERIALS AND METHODS ^ OBSERVATIONS 6 Five-Somi te Stag© . 6 Seven-Somite Stage 8 Eight-Somite Stage 9 Ten- and bleven-Somite Stage 12 Twe 1 ve-Somi te Stage • \\i Fifteen-Somite Stage 18 Seventeen-Somite Stage 21 Eighteen-Somite Stage 2$ Twenty- and Twenty- one -Somite Stage 27 INTERPRETATIONS AND DISCUSSION 30 Vasculogenesis • 30 Cardiogenesis 33 The Origin and Development of Arteries \ 3lj. Aortic Arches •••« 3I4. Cranial Arterie s ...•• 36 The Dorsal Aorta 37 Intersegmental AAteries 39 Vertebral Arteries 39 Vitelline Arteries }±q The Allantoic Artery \±\ Ill IITERPRETATION AND DISCUSSION (Contd.) The Origin and Development of Veins •• kl The Anterior Cardinal Veins . I4.I Posterior Cardinal Veins k2 Umbilical Veins U3 Common Cardinal Veins kh Interconnecting Vessels Ui> SUMMARY kl LITERA°URE CITED $1 ACKNOWLEDGMENTS 53 APPENDIX 5U HTmDUCTIOW AND LITFRATORF. rfvibw While the dog has been employed extensively as a labora- tory animal in various fields of scientific endeavour, the use of this animal in embryology has been neglected. As a con- sequence, the literature on the circulatory system of the dog was represented only by an unpublished thesis by Duffey (3) on oardlogenesis and the first heart movements. -

Development-Of-Heart
ANATOMY EMBRYOLOGY EMBRYOLOGY DEVLOPMENT OF THE HEART Normal Heart Structure Heart Anatomy HEART Normal Anatomy Contents Development of the Heart Tube Derivatives of the Heart Tube Cardiac Looping Development Heart Partition of The Heart Formation of Cardiac Valves Formation of AV Septum Foetal Circulation Formation of Aortic Arch System The cardiovascular system is the first system to function in the Embryo. Starts developing in the middle of the third week, and heart becomes functional at the beginning of fourth week Development The cells of the splanchnic of Heart mesoderm in the cardiogenic area proliferate to form the angiogenetic clusters. The canalization of angiogenetic clusters in the splanchnic mesoderm of cardiogenic area results in the formation of the paired heart tubes. Day 16 heart heart field (PHF). progenitor PHF gives rise to cells atria, migrate to area cranial to left ventricle and neural part of right folds, horseshoe- ventricle shaped region of Secondary heart Development splanchnic field (SHF) forms lateral of the Heart plate the mesoderm. This is remainder of R. primary Ventricle, Conus Cordis Truncus Arteriosus Development of the Heart Diagrammatic Development of the Heart Development of the Heart Development of the Heart Tube 1. Foregut 2. Intraembryonic coelom 3. Heart tubes 4. Dorsal mesocardium 5. Epimyocardium 6. Neural groove 7. Neural crest 8. Notochord 9. Dorsal aorta SHF lies posterior to Pharynx in splanchnic mesoderm, is regulated by neural crest cells that migrate through pharyngeal arches in this region -

Cardiovascular System Note: the Cardiovascular System Develops Early (Week 3), Enabling the Embryo to Grow Beyond the Short
Lymphatics: Lymph vessel formation is similar to blood angiogenesis. Lymphatics begin as lymph sacs in three regions: jugular (near brachiocephalic veins); cranial abdominal (future cysterna chyla); and iliac region. Lym- phatic vessels (ducts) form as outgrowths of the sacs. mesenchyme Lymph nodes are produced by localized mesoder- sinusoid lymph duct lumen mal invaginations that partition the vessel lumen into sinu- soids. The mesoderm develops a reticular framework within which mesodermal lymphocytes accumulate. The spleen and hemal nodes (in ruminants) invagination develop similar to the way lymph nodes develop. Lymph Node Formation Prior to birth, fetal circulation is designed for an in utero aqueous environment where the pla- centa oxygenates fetal blood. Suddenly, at birth... Three In-Utero Adjustments ductus Stretching and constriction of arteriosus umbilical arteries shifts fetal blood flow aortic arch from the placenta to the fetus. Reduced pulmonary trunk L atrium venous return through the (left) umbili- foramen ovale R cal vein and ductus venosus allows the atrium latter to gradually close (over a period caudal vena cava of days). Bradykinin released by expand- ductus venosus ing lungs and increased oxygen concen- tration in blood triggers constriction of aorta the ductus arteriosus which, over two liver months, is gradually converted to a fibrous structure, the ligamentum arte- umbilical v. riosum. portal v. The increased blood flow to the lungs and then to the left atrium equalizes pres- sure in the two atria, resulting in closure umbilical aa. of the foramen ovale that eventually grows permanent. 29 The cardiogenic area, the place where the embryonic heart originates, is located . -

Dosage-Sensitive Requirement for Mouse Dll4 in Artery Development
Downloaded from genesdev.cshlp.org on September 26, 2021 - Published by Cold Spring Harbor Laboratory Press RESEARCH COMMUNICATION Dosage-sensitive requirement 2000), which were also observed, to a lesser degree, in Hey1/Hey2 double mutants, the putative downstream for mouse Dll4 targets of Notch signaling (Fischer et al. 2004). Here we in artery development show, in contrast, that the Dll4 ligand alone is required in a dosage-sensitive manner for normal arterial pattern- António Duarte,1,5 Masanori Hirashima,3,5,6 ing in development. Rui Benedito,1 Alexandre Trindade,1 The Dll4 gene was inactivated by targeted disruption 1 2 1 in embryonic stem (ES) cells. The targeting vector was Patrícia Diniz, Evguenia Bekman, Luís Costa, designed to replace the initiation codon and the first 2 3,4,7 Domingos Henrique, and Janet Rossant three coding exons with the -galactosidase (lacZ) re- porter gene (Fig. 1A). Electroporation of R1 ES cells with 1CIISA, Faculdade de Medicina Veterina´ria, 1300-0-477 the targeting vector followed by G418 selection resulted Lisboa, Portugal; 2Instituto de Medicina Molecular, Faculdade in the derivation of four correctly gene-targeted ES cell de Medicina, 1649-9-028 Lisboa, Portugal; 3Samuel Lunenfeld lines. Chimeric mice were generated by aggregation be- Research Institute, Mount Sinai Hospital, Toronto, Ontario, tween Dll4+/− ES cells and ICR host embryos. When Canada M5G1X5; 4Department of Molecular and Medical crossed with wild-type ICR females, five male chimeras Genetics, University of Toronto, Toronto, Ontario, produced F1 agouti pups at 100% frequency. Genotyping Canada M5S 1A8 of F1 agouti mice by Southern blotting or polymerase +/− Involvement of the Notch signaling pathway in vascular chain reaction (PCR; Fig. -

LAC.12 Embryology 2019-2020 Dr.Mahdi Alheety
LAC.12 Embryology 2019-2020 Dr.Mahdi ALheety Cardiovascular System Establishment of the Cardiogenic Field The vascular system appears in the middle of the third week, when the embryo is no longer able to satisfy its nutritional requirements by diffusion alone. Progenitor heart cells lie in the epiblast, immediately adjacent to the cranial end of the primitive streak. From there, they migrate through the streak and into the splanchnic layer of lateral plate mesoderm where they form a horseshoe-shaped cluster of cells called the primary heart field (PHF) cranial to the neural folds. As the progenitor heart cells migrate and form the PHF during days 16 to18, they are specified on both sides from lateral to medial to become the atria, left ventricle, and most of the right ventricle. Patterning of these cells occurs at the same time that laterality (left-right sidedness) is being established for the entire embryo and this process and the signaling pathway it is dependent upon is essential for normal heart development. The remainder of the heart, including part of the right ventricle and outflow tract (conus cordis and truncus arteriosus), is derived from the secondary heart field (SHF). This field of cells appears slightly later (days 20 to 21) than those in the PHF, resides in splanchnic mesoderm ventral to the posterior pharynx, and is responsible for lengthening the outflow tract. Cells in the SHF also exhibit laterality, such that those on the right side contribute to the left of the outflow tract region and those on the left contribute to the right. -
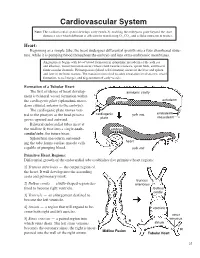
Cardiovascular System Note: the Cardiovascular System Develops Early (Week-3), Enabling the Embryo to Grow Beyond the Short
Cardiovascular System Note: The cardiovascular system develops early (week-3), enabling the embryo to grow beyond the short distances over which diffusion is efficient for transferring 2O , CO2, and cellular nutrients & wastes. Heart: Beginning as a simple tube, the heart undergoes differential growth into a four chambered struc- ture, while it is pumping blood throughout the embryo and into extra-embryonic membranes. Angiogenesis begins with blood island formation in splanchnic mesoderm of the yolk sac and allantois. Vessel formation occurs when island vesicles coalesce, sprout buds, and fuse to form vascular channels. Hematopoiesis (blood cell formation) occurs in the liver and spleen and later in the bone marrow. The transition from fetal to adult circulation involves new vessel formation, vessel merger, and degeneration of early vessels. Formation of a Tubular Heart: The first evidence of heart develop- amnionic cavity ment is bilateral vessel formation within ectoderm the cardiogenic plate (splanchnic meso- embryo derm situated anterior to the embryo). The cardiogenic plate moves ven- tral to the pharynx as the head process cardiogenic yolk sac endoderm mesoderm grows upward and outward. plate Bilateral endocardial tubes meet at the midline & fuse into a single endo- embryo cardial tube, the future heart. Splanchnic mesoderm surround- ing the tube forms cardiac muscle cells heart capable of pumping blood. yolk sac Primitive Heart Regions: Differential growth of the endocardial tube establishes five primitive heart regions: 1] Truncus arteriosus — the output region of the heart. It will develop into the ascending aorta and pulmonary trunk. truncus 2] Bulbus cordis — a bulb-shaped region des- arteriosus tined to become right ventricle. -

Embryonic Adaptations
Chapter 7 Embryonic Adaptations mbryonic Adaptations are organs which are not part of the embryo proper, but serve E to bring nutrients & oxygen to the embryo and to store waste products. 7.1. Formation of Peripheral Circulation in the ChickChick Embryo During the 2nd & 3rd days of incubation the inner part of the area opaca develops a series of blood vessels. This region of the area opaca becomes known as the area vasculosa . The outer part of the area opaca is known as the area vitellina . The development of area vasculosa blood vessels occurs as follows: Groups of densely packed mesodermal cells, called blood islands , appear in the area opaca . The cells of the blood islands form blood cells centrally and an epithelial layer around the periphery. The blood islands establish communications with one another to form a network of blood vessels. These blood vessels are all ultimately connected to the terminal sinus . {B283} The terminal sinus is a large blood vessel which runs around the periphery of the area vasculosa . The area vasculosa blood vessels connect with vessels of the embryo proper. The left & right vitelline veins of the area vasculosa are continuous with the ductus (sinus) venosus of the embryo. The left & right vitelline arteries of the area vasculosa join the dorsal aorta of the embryo. By 60 hours of incubation the heart begins to beat and blood circulates to and from the yolk. Fig.7.1. Chick Embryo 7.2. The Amniotic Egg The Amphibian egg is covered with a simple membrane and must be deposited in water (or a moist environment) or it will dry out. -

Cardiogenesis in the Bovine to 35 Somites
CARDIOGENESIS IN THE BOVINE TO 35 SOMITES by PATRICIA ANN NODEN A. A., Chanute Junior College, 1962 B. S., Kansas State University, 1964 A MASTER'S THESIS submitted in partial fulfillment of the requirements for the degree MASTER OF SCIENCE Department of Zoology KANSAS STATE UNIVERSITY Manhattan, Kansas 1966 Approved by: ii N7G> TABLE OF CONTENTS Oocu mtA t INTRODUCTION 1 METHODS AND MATERIALS 6 OBSERVATIONS 9 Presomite Stage 9 Six Somite Stage 9 Nine Somite Stage 10 13 Somite Stage 18 19 Somite Stage 18 22 Somite Stage 19 24-25 Somite Stage 20 28-30 Somite Stage ....... 21 33-35 Somite Stage 24 DISCUSSION 27 CONCLUSION S3 ACKNOWLEDGMENTS 35 BIBLIOGRAPHY . 36 . INTRODUCTION Mammalian cardiogenesis is a vast field which so far has not been thoroughly explored. There are few species in which the complete development of the heart has been studied and many in which partial formation has been observed The formation of the heart to the functional stage in the dog (14 somites) was studied by Bonnet (1901) and Duffey (1953). Duffey's thesis was used exclusively for comparison in this paper. The rat has been studied in some detail, from presomite to birth and after, by Burlingame and Long (1939) and early stages by Ravn (1895) and Goss (1942, 1952). The tubular phase of cardiogenesis in the rabbit of 10-13 somites was described by Girgis (1930, 1933). Dwinnel (1939) worked with early somite stages. Development of the aortic arches was described by Bremer (1912). Hensen (1875), Bom (1888, 1889), Strahl and Carius (1889) and Rouviere (1904) have described early cardiac formation. -

EXTRA-EMBRYONIC MEMBRANES in CHICK Yolksac, Yolk Sac (Gut); Amnion and Chorion (Body Wall) and Allantois (Primitive Bladder). Al
EXTRA-EMBRYONIC MEMBRANES IN CHICK The history of the foetal-membranes in chick is with the of an correlated presence anormous mass of and an yolk embryonic life within a shell. the a spent Although, original blastoderm is small disc, it spreads by peripheral and covers the surface of the But growth eventually entire egg. only the most central region is directly concerned with the formation of the The is embryo proper. remaining blastoderm extra-embryonic and it is this portion that furnishes the embryonic or foetal membranes. These which arise are auxillary organs partly for protection of the embryo, and more especially to provide for nutrition, respiration and excretion until the independent existence is attained. its The fundamental set of embryonic membranes in chick and other amniotes includes the yolksac, amnion, chorion (serosa in mammals) and allantois which have evolved in relation to the structures that were already in existence following Yolk sac (gut); amnion and chorion (body wall) and allantois (primitive bladder). All these extra-embryonic membranous sacs are composite structures and involve two germ The layers. amnion and chorion are composeed of extra-embryonic ectoderm and somatic layer of mesoderm (somatopleure) whiler the yolk sac and allantois are composed of extra-embryonic endoderm and splanchnic layer of mesoderm (splanchnopleure), separated by a space which is extra-embryonic coelom. These components are continuous with their counterparts in the embryonic teritory of the blastoderm.. 1. Yolk Sac Yolk sac is the first of the extra-embryonic membranes to make its appearance. The splanchnopleure of the chick, instead of forming a closed gut, grows over the yolk surface as extra-embryonic extension and eventually encloses the entire yok to form the yolk sae, The primitive gut has a cellular wall dorsally only, with the yolk acting as a temporary floor (Fig. -

Flow Regulates Arterial-Venous Differentiation in the Chick Embryo
Development ePress online publication date 17 December 2003 Research article 361 Flow regulates arterial-venous differentiation in the chick embryo yolk sac Ferdinand le Noble1,2, Delphine Moyon2, Luc Pardanaud2, Li Yuan2, Valentin Djonov3, Robert Matthijsen1, Christiane Bréant2, Vincent Fleury4 and Anne Eichmann2,* 1Department of Physiology, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, The Netherlands 2Inserm U36, Collège de France, 11, Place Marcelin Berthelot, 75005 Paris, France 3Department of Anatomy, University of Bern, Switzerland 4Department of Physics, Ecole Polytechnique, Palaiseau, France *Author for correspondence (e-mail: [email protected]) Accepted 17 October 2003 Development 131, 361-375 Published by The Company of Biologists 2004 doi:10.1242/dev.00929 Summary Formation of the yolk sac vascular system and its growth of veins. Arterial-venous differentiation and connection to the embryonic circulation is crucial for patterning are controlled by hemodynamic forces, as shown embryo survival in both mammals and birds. Most mice by flow manipulation and in situ hybridization with arterial with mutations in genes involved in vascular development markers ephrinB2 and neuropilin 1, which show that die because of a failure to establish this circulatory loop. expression of both mRNAs is not genetically determined Surprisingly, formation of yolk sac arteries and veins has but plastic and regulated by flow. In vivo application of not been well described in the recent literature. Using time- ephrinB2 or EphB4 in the developing yolk sac failed to lapse video-microscopy, we have studied arterial-venous produce any morphological effects. By contrast, ephrinB2 differentiation in the yolk sac of chick embryos. and EphB4 application in the allantois of older embryos Immediately after the onset of perfusion, the yolk sac resulted in the rapid formation of arterial-venous shunts.