Cardiogenesis in the Bovine to 35 Somites
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Evolving Cardiac Lymphatic Vasculature in Development, Repair and Regeneration
REVIEWS The evolving cardiac lymphatic vasculature in development, repair and regeneration Konstantinos Klaourakis 1,2, Joaquim M. Vieira 1,2,3 ✉ and Paul R. Riley 1,2,3 ✉ Abstract | The lymphatic vasculature has an essential role in maintaining normal fluid balance in tissues and modulating the inflammatory response to injury or pathogens. Disruption of normal development or function of lymphatic vessels can have severe consequences. In the heart, reduced lymphatic function can lead to myocardial oedema and persistent inflammation. Macrophages, which are phagocytic cells of the innate immune system, contribute to cardiac development and to fibrotic repair and regeneration of cardiac tissue after myocardial infarction. In this Review, we discuss the cardiac lymphatic vasculature with a focus on developments over the past 5 years arising from the study of mammalian and zebrafish model organisms. In addition, we examine the interplay between the cardiac lymphatics and macrophages during fibrotic repair and regeneration after myocardial infarction. Finally, we discuss the therapeutic potential of targeting the cardiac lymphatic network to regulate immune cell content and alleviate inflammation in patients with ischaemic heart disease. The circulatory system of vertebrates is composed of two after MI. In this Review, we summarize the current complementary vasculatures, the blood and lymphatic knowledge on the development, structure and function vascular systems1. The blood vasculature is a closed sys- of the cardiac lymphatic vasculature, with an emphasis tem responsible for transporting gases, fluids, nutrients, on breakthroughs over the past 5 years in the study of metabolites and cells to the tissues2. This extravasation of cardiac lymphatic heterogeneity in mice and zebrafish. -

Glossary of Key Terms and Concepts - Chapter 8
Glossary of Key Terms and Concepts - Chapter 8 Angioblasts - These "vessel-forming cells" may arise from any kind of mesoderm except prechordal plate mesoderm. Angioblastic cords - Angiocysts coalesce to form short blind-ended angioblastic cords. Angioblastic plexuses - Angioblastic cords coalesce to form complex interconnected vascular networks or plexuses. Angiocysts - These vesicles are formed by angioblasts during the process of vasculogenesis. Angiogenesis - This is the mechanism whereby preexisting vessels lengthen or branch by sprouting. Aortic arches - These vessels have been modified in humans to form the great vessels of the thorax (also see Ch. 7). Axis arteries - These central arteries of the limbs are derived from the 7th intersegmental arteries (upper limb) and 5th lumbar intersegmental arteries (lower limb). Blood islands - Blood islands are cysts of angioblasts containing hemoblasts. These coalesce to form blood vessels in the yolk sac and also form the coronary vasculature. Branchial arches - These are the gill bars of fish. Homologous structures of humans are more appropriately named "pharyngeal" arches. Cardinal system of veins - These veins drain the head and neck and body wall and extremities of the embryo. Anterior cardinals drain the head and neck and the trunk and lower extremities are drained by paired posterior cardinals. The posterior cardinal veins are replaced by subcardinal and supracardinal veins during the second month. Coronary vessels - These vessels of the heart form from epicardium as subepicardial plexuses fuse with sprouts of the aorta and coronary sinus to form the coronary arteries and coronary veins respectively. Endothelial cells - These cells arise from angioblasts to form the initial vascular network. -

Azygos Vein System Abnormality: Case Report
Gülhane Týp Dergisi 2006; 48: 180-182 OLGU SUNUMU © Gülhane Askeri Týp Akademisi 2006 Azygos vein system abnormality: case report Necdet Kocabýyýk (*), Tunç Kutoðlu (**), Soner Albay (*), Bülent Yalçýn (*), Hasan Ozan (*) Summary Introduction Variations seen in the thoracic vein system are Abnormalities related to the azygos system are not rare (1). In a series related to the development of these veins. of 200 cases, Bergman et al. have reported the incidence of this anomaly During the dissection from the posterior medi- astinum of the 60-year-old male cadaver, it 26% (2). These abnormalities are generally explained by the embryolog- was observed that there was no complete ical development. Venous branching of the azygos vein varies (3). There accessory hemiazygos vein, and both posterior are two origins of the azygos and hemiazygos veins. By union of these intercostal veins and hemiazygos vein (above origins and regression of some parts, azygos system comes into its final T10 level) drained bilaterally to the azygos vein. Considering these types of variations is status (4). Different types of structures may occur when these veins important during imaging this region and surgi- develop. Abnormalities about azygos system and especially the variations cal operations. of the hemiazygos veins are not clearly described in the literature. In this Key words: Azygos vein, hemiazygos vein, superior vena cava, venous anomaly presentation absence of the accessory hemiazygos vein and possible causes of these types of variations are discussed in view of the embry- Özet ological development. Azigos ven sistem anomalisi: olgu sunumu Toraks ven sisteminde görülen varyasyonlar, embriyolojik olarak bu venlerin geliþimiyle ilgi- Case Report lidir. -

Cardiogenesis with a Focus on Vasculogenesis and Angiogenesis
Received: 27 August 2019 | Revised: 4 February 2020 | Accepted: 20 February 2020 DOI: 10.1111/ahe.12549 SPECIAL ISSUE Cardiogenesis with a focus on vasculogenesis and angiogenesis Katrin Borasch1 | Kenneth Richardson2 | Johanna Plendl1 1Department of Veterinary Medicine, Institute of Veterinary Anatomy, Freie Abstract University Berlin, Berlin, Germany The initial intraembryonic vasculogenesis occurs in the cardiogenic mesoderm. Here, 2 College of Veterinary Medicine, School a cell population of proendocardial cells detaches from the mesoderm that subse- of Veterinary and Life Sciences, Murdoch University, Murdoch, WA, Australia quently generates the single endocardial tube by forming vascular plexuses. In the course of embryogenesis, the endocardium retains vasculogenic, angiogenic and Correspondence Johanna Plendl, Department of Veterinary haematopoietic potential. The coronary blood vessels that sustain the rapidly ex- Medicine, Institute of Veterinary Anatomy, panding myocardium develop in the course of the formation of the cardiac loop by Freie University Berlin, Berlin, Germany. Email: [email protected] vasculogenesis and angiogenesis from progenitor cells of the proepicardial serosa at the venous pole of the heart as well as from the endocardium and endothelial cells of Funding information Freie Universität Berlin the sinus venosus. Prospective coronary endothelial cells and progenitor cells of the coronary blood vessel walls (smooth muscle cells, perivascular cells) originate from different cell populations that are in close spatial as well as regulatory connection with each other. Vasculo- and angiogenesis of the coronary blood vessels are for a large part regulated by the epicardium and epicardium-derived cells. Vasculogenic and angiogenic signalling pathways include the vascular endothelial growth factors, the angiopoietins and the fibroblast growth factors and their receptors. -

Vertebrate Embryos As Tools for Anti-Angiogenic Drug Screening and Function
Vertebrate embryos as tools for anti-angiogenic drug screening and function Shaunna Beedie1,2, Alexandra J. Diamond1, Lucas Rosa Fraga1, William D. Figg2, Neil Vargesson1, * 1 School of Medicine, Medical Sciences and Nutrition, Institute of Medical Sciences, University of Aberdeen, Foresterhill, Aberdeen, UK. 2 Molecular Pharmacology Section, Genitourinary Malignancies Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health. Bethesda. USA. *Corresponding author Neil Vargesson: [email protected]; [email protected] Key words: angiogenesis, chicken, zebrafish, mouse, rat, rabbit, non-human primates, thalidomide Abstract The development of new angiogenic inhibitors highlights a need for robust screening assays that adequately capture the complexity of vessel formation, and allow for the quantitative evaluation of the teratogenicity of new anti- angiogenic agents. This review discusses the use of screening assays in vertebrate embryos, specifically focusing upon chicken and zebrafish embryos, for the detection of anti-angiogenic agents. Introduction and background The cardiovascular system is vital for normal embryonic development in utero [1]. It is one of the earliest differentiating and functioning organ systems, emphasizing its importance to the embryo [2-7]. The primitive vascular system develops by vasculogenesis, de novo differentiation and growth of vessels from the mesoderm. The major vessels in the embryo form first, the dorsal aortae (transporting oxygenated blood from the placenta or yolk sac to the heart) and vena cava or vitelline veins (transporting deoxygenated blood back to the heart or yolk sac) develop by vasculogenesis. Expansion of the nascent vascular network can then occur by angiogenesis, the process of vessel formation from the preexisting vasculature. -

Cardiovascular System Heart Development Cardiovascular System Heart Development
Cardiovascular System Heart Development Cardiovascular System Heart Development In human embryos, the heart begins to beat at approximately 22-23 days, with blood flow beginning in the 4th week. The heart is one of the earliest differentiating and functioning organs. • This emphasizes the critical nature of the heart in distributing blood through the vessels and the vital exchange of nutrients, oxygen, and wastes between the developing baby and the mother. • Therefore, the first system that completes its development in the embryo is called cardiovascular system. https://www.slideshare.net/DrSherifFahmy/intraembryonic-mesoderm-general-embryology Mesoderm is one of the three • Connective tissue primary germ layers that • Smooth and striated muscle • Cardiovascular System differentiates early in • Kidneys development that collectively • Spleen • Genital organs, ducts gives rise to all subsequent • Adrenal gland cortex tissues and organs. The cardiovascular system begins to develop in the third week of gestation. Blood islands develop in the newly formed mesoderm, and consist of (a) a central group of haemoblasts, the embryonic precursors of blood cells; (b) endothelial cells. Development of the heart and vascular system is often described together as the cardiovascular system. Development begins very early in mesoderm both within (embryonic) and outside (extra embryonic, vitelline, umblical and placental) the embryo. Vascular development occurs in many places. • Blood islands coalesce to form a vascular plexus. Preferential channels form arteries and veins. • Day 17 - Blood islands form first in the extra-embryonic mesoderm • Day 18 - Blood islands form next in the intra-embryonic mesoderm • Day 19 - Blood islands form in the cardiogenic mesoderm and coalesce to form a pair of endothelial heart tubes Development of a circulation • A circulation is established during the 4th week after the myocardium is differentiated. -
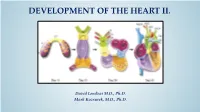
Development of Right Ventricle
DEVELOPMENT OF THE HEART II. David Lendvai M.D., Ph.D. Mark Kozsurek, M.D., Ph.D. • Septation of the common atrioventricular (AV) orifice. • Formation of the interatrial septum. • Formation of the muscular interventricular septum. • Appearance of the membranous interventricular septum and the spiral aorticopulmonary septum. right left septum primum septum primum septum primum septum primum septum primum septum primum foramen primum foramen primum septum primum septum primum foramen primum foramen primum septum primum septum primum foramen secundum foramen secundum foramen primum foramen primum septum primum foramen secundum septum primum foramen secundum foramen primum foramen primum septum primum septum primum foramen secundum foramen secundum septum secundum septum secundum foramen secundum foramen ovale foramen ovale septum primum septum primum septum secundum septum secundum foramen secundum foramen ovale foramen ovale septum primum septum primum septum secundum septum secundum foramen secundum septum primum foramen ovale foramen ovale septum primum SUMMARY • The septation of the common atrium starts with the appearance of the crescent-shaped septum primum. The opening of this septum, the foramen primum, becomes progressively smaller. • Before the foramen primum completly closes, postero-superiorly several small openings appear on the septum primum. These perforations coalesce later and form the foramen secundum. • On the right side of the septum primum a new septum, the septum secundum, starts to grow. The orifice of the septum secundum is the foramen ovale. • Finally two crescent-like, incomplete, partially overlapping septa exist with one hole on each. Septum secundum is more rigid and the septum primum on its left side acts as a valve letting the blood flow exclusively from the right to the left. -

Abnormalities of Placental Development and Function Are
bioRxiv preprint doi: https://doi.org/10.1101/388074; this version posted August 27, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abnormalities of placental development and function are associated with the different fetal growth patterns of hypoplastic left heart syndrome and transposition of the great arteries. Weston Troja1, Kathryn J. Owens1, Jennifer Courtney1, Andrea C. Hinton3, Robert B. Hinton3, James F. Cnota2 and Helen N. Jones1* 1. Center for Fetal and Placental Therapy, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, Cincinnati, Ohio 2. Heart Institute, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, Cincinnati, Ohio 3. TriHealth Maternal Fetal Medicine, 375 Dixsmyth Ave, Cincinnati Ohio *Corresponding Author: Helen N. Jones [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/388074; this version posted August 27, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Background: Birthweight is a critical predictor of congenital heart disease (CHD) surgical outcomes. Hypoplastic left heart syndrome (HLHS) is cyanotic CHD with known fetal growth restriction and placental abnormalities. Transposition of the great arteries (TGA) is cyanotic CHD with normal fetal growth. Comparison of the placenta in these diagnoses may provide insights on the fetal growth abnormality of CHD. Methods: Clinical data and placental histology from placentas associated with Transposition of the Great Arteries (TGA) were analyzed for gross pathology, morphology, maturity and vascularity and compared to both control and previously analyzed HLHS placentas [1]. -
Endothelial Cell Dynamics in Vascular Development: Insights from Live-Imaging in Zebrafish
fphys-11-00842 July 20, 2020 Time: 12:10 # 1 REVIEW published: 22 July 2020 doi: 10.3389/fphys.2020.00842 Endothelial Cell Dynamics in Vascular Development: Insights From Live-Imaging in Zebrafish Kazuhide S. Okuda1,2 and Benjamin M. Hogan1,2,3* 1 Organogenesis and Cancer Program, Peter MacCallum Cancer Centre, Melbourne, VIC, Australia, 2 Sir Peter MacCallum Department of Oncology, University of Melbourne, Melbourne, VIC, Australia, 3 Department of Anatomy and Neuroscience, University of Melbourne, Melbourne, VIC, Australia The formation of the vertebrate vasculature involves the acquisition of endothelial cell identities, sprouting, migration, remodeling and maturation of functional vessel networks. To understand the cellular and molecular processes that drive vascular development, live-imaging of dynamic cellular events in the zebrafish embryo have proven highly informative. This review focusses on recent advances, new tools and new insights from imaging studies in vascular cell biology using zebrafish as a model system. Keywords: vasculogenesis, angiogenesis, lymphangiogenesis, anastomosis, endothelial cell, zebrafish Edited by: INTRODUCTION Elizabeth Anne Vincent Jones, KU Leuven, Belgium Vascular development is an early and essential process in the formation of a viable vertebrate Reviewed by: embryo. Blood and lymphatic vascular networks are composed of a complex mix of cell types: for Anna Rita Cantelmo, example smooth muscle cells, fibroblasts, pericytes and immune cells are intimately associated and Université Lille Nord de France, even integrated within mature vessel walls (Rouget, 1873; Horstmann, 1952; Nicosia and Madri, France 1987; Fantin et al., 2010; Gordon et al., 2010). The inner lining of the vasculature, made up of Jingjing Zhang, endothelial cells (ECs), is the earliest part of the vasculature to develop in the embryo and is Affiliated Hospital of Guangdong Medical University, China instructive in recruiting other lineages and cell types as the vascular system matures. -

Development of the Vascular System in Five to Twenty-One
THE DEVELOPMENT OF THE VASCULAR SYSTEM IN FIVE TO TWtNTY-ONE SOMITE DOG EMBRYOS by ELDEN WILLIAM MARTIN B, S., Kansas State College of Agriculture and ADolied Science, 195>U A THESIS submitted in partial fulfillment of the requirements for the degree MASTER OF SCIENCE Department of Zoology KANSAS STATV: COLLEGE OF AGRICULTURE AND A PLIED SCIENCE 1958 LP TH Ooco/*>*Tv TABLE OF CONTENTS INTRO IXJ CTION AND LITERATURE REVIEW 1 MATERIALS AND METHODS ^ OBSERVATIONS 6 Five-Somi te Stag© . 6 Seven-Somite Stage 8 Eight-Somite Stage 9 Ten- and bleven-Somite Stage 12 Twe 1 ve-Somi te Stage • \\i Fifteen-Somite Stage 18 Seventeen-Somite Stage 21 Eighteen-Somite Stage 2$ Twenty- and Twenty- one -Somite Stage 27 INTERPRETATIONS AND DISCUSSION 30 Vasculogenesis • 30 Cardiogenesis 33 The Origin and Development of Arteries \ 3lj. Aortic Arches •••« 3I4. Cranial Arterie s ...•• 36 The Dorsal Aorta 37 Intersegmental AAteries 39 Vertebral Arteries 39 Vitelline Arteries }±q The Allantoic Artery \±\ Ill IITERPRETATION AND DISCUSSION (Contd.) The Origin and Development of Veins •• kl The Anterior Cardinal Veins . I4.I Posterior Cardinal Veins k2 Umbilical Veins U3 Common Cardinal Veins kh Interconnecting Vessels Ui> SUMMARY kl LITERA°URE CITED $1 ACKNOWLEDGMENTS 53 APPENDIX 5U HTmDUCTIOW AND LITFRATORF. rfvibw While the dog has been employed extensively as a labora- tory animal in various fields of scientific endeavour, the use of this animal in embryology has been neglected. As a con- sequence, the literature on the circulatory system of the dog was represented only by an unpublished thesis by Duffey (3) on oardlogenesis and the first heart movements. -

Development-Of-Heart
ANATOMY EMBRYOLOGY EMBRYOLOGY DEVLOPMENT OF THE HEART Normal Heart Structure Heart Anatomy HEART Normal Anatomy Contents Development of the Heart Tube Derivatives of the Heart Tube Cardiac Looping Development Heart Partition of The Heart Formation of Cardiac Valves Formation of AV Septum Foetal Circulation Formation of Aortic Arch System The cardiovascular system is the first system to function in the Embryo. Starts developing in the middle of the third week, and heart becomes functional at the beginning of fourth week Development The cells of the splanchnic of Heart mesoderm in the cardiogenic area proliferate to form the angiogenetic clusters. The canalization of angiogenetic clusters in the splanchnic mesoderm of cardiogenic area results in the formation of the paired heart tubes. Day 16 heart heart field (PHF). progenitor PHF gives rise to cells atria, migrate to area cranial to left ventricle and neural part of right folds, horseshoe- ventricle shaped region of Secondary heart Development splanchnic field (SHF) forms lateral of the Heart plate the mesoderm. This is remainder of R. primary Ventricle, Conus Cordis Truncus Arteriosus Development of the Heart Diagrammatic Development of the Heart Development of the Heart Development of the Heart Tube 1. Foregut 2. Intraembryonic coelom 3. Heart tubes 4. Dorsal mesocardium 5. Epimyocardium 6. Neural groove 7. Neural crest 8. Notochord 9. Dorsal aorta SHF lies posterior to Pharynx in splanchnic mesoderm, is regulated by neural crest cells that migrate through pharyngeal arches in this region -

Cardiovascular System Note: the Cardiovascular System Develops Early (Week 3), Enabling the Embryo to Grow Beyond the Short
Lymphatics: Lymph vessel formation is similar to blood angiogenesis. Lymphatics begin as lymph sacs in three regions: jugular (near brachiocephalic veins); cranial abdominal (future cysterna chyla); and iliac region. Lym- phatic vessels (ducts) form as outgrowths of the sacs. mesenchyme Lymph nodes are produced by localized mesoder- sinusoid lymph duct lumen mal invaginations that partition the vessel lumen into sinu- soids. The mesoderm develops a reticular framework within which mesodermal lymphocytes accumulate. The spleen and hemal nodes (in ruminants) invagination develop similar to the way lymph nodes develop. Lymph Node Formation Prior to birth, fetal circulation is designed for an in utero aqueous environment where the pla- centa oxygenates fetal blood. Suddenly, at birth... Three In-Utero Adjustments ductus Stretching and constriction of arteriosus umbilical arteries shifts fetal blood flow aortic arch from the placenta to the fetus. Reduced pulmonary trunk L atrium venous return through the (left) umbili- foramen ovale R cal vein and ductus venosus allows the atrium latter to gradually close (over a period caudal vena cava of days). Bradykinin released by expand- ductus venosus ing lungs and increased oxygen concen- tration in blood triggers constriction of aorta the ductus arteriosus which, over two liver months, is gradually converted to a fibrous structure, the ligamentum arte- umbilical v. riosum. portal v. The increased blood flow to the lungs and then to the left atrium equalizes pres- sure in the two atria, resulting in closure umbilical aa. of the foramen ovale that eventually grows permanent. 29 The cardiogenic area, the place where the embryonic heart originates, is located .