Conserved Signaling Mechanisms in Drosophila Heart Development
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Functional Analysis of the Homeobox Gene Tur-2 During Mouse Embryogenesis
Functional Analysis of The Homeobox Gene Tur-2 During Mouse Embryogenesis Shao Jun Tang A thesis submitted in conformity with the requirements for the Degree of Doctor of Philosophy Graduate Department of Molecular and Medical Genetics University of Toronto March, 1998 Copyright by Shao Jun Tang (1998) National Library Bibriothèque nationale du Canada Acquisitions and Acquisitions et Bibiiographic Services seMces bibliographiques 395 Wellington Street 395, rue Weifington OtbawaON K1AW OttawaON KYAON4 Canada Canada The author has granted a non- L'auteur a accordé une licence non exclusive licence alIowing the exclusive permettant à la National Library of Canada to Bibliothèque nationale du Canada de reproduce, loan, distri%uteor sell reproduire, prêter' distribuer ou copies of this thesis in microform, vendre des copies de cette thèse sous paper or electronic formats. la forme de microfiche/nlm, de reproduction sur papier ou sur format électronique. The author retains ownership of the L'auteur conserve la propriété du copyright in this thesis. Neither the droit d'auteur qui protège cette thèse. thesis nor substantial extracts fkom it Ni la thèse ni des extraits substantiels may be printed or otherwise de celle-ci ne doivent être imprimés reproduced without the author's ou autrement reproduits sans son permission. autorisation. Functional Analysis of The Homeobox Gene TLr-2 During Mouse Embryogenesis Doctor of Philosophy (1998) Shao Jun Tang Graduate Department of Moiecular and Medicd Genetics University of Toronto Abstract This thesis describes the clonhg of the TLx-2 homeobox gene, the determination of its developmental expression, the characterization of its fiuiction in mouse mesodem and penpheral nervous system (PNS) developrnent, the regulation of nx-2 expression in the early mouse embryo by BMP signalling, and the modulation of the function of nX-2 protein by the 14-3-3 signalling protein during neural development. -

Fetal Blood Flow and Genetic Mutations in Conotruncal Congenital Heart Disease
Journal of Cardiovascular Development and Disease Review Fetal Blood Flow and Genetic Mutations in Conotruncal Congenital Heart Disease Laura A. Dyer 1 and Sandra Rugonyi 2,* 1 Department of Biology, University of Portland, Portland, OR 97203, USA; [email protected] 2 Department of Biomedical Engineering, Oregon Health & Science University, Portland, OR 97239, USA * Correspondence: [email protected] Abstract: In congenital heart disease, the presence of structural defects affects blood flow in the heart and circulation. However, because the fetal circulation bypasses the lungs, fetuses with cyanotic heart defects can survive in utero but need prompt intervention to survive after birth. Tetralogy of Fallot and persistent truncus arteriosus are two of the most significant conotruncal heart defects. In both defects, blood access to the lungs is restricted or non-existent, and babies with these critical conditions need intervention right after birth. While there are known genetic mutations that lead to these critical heart defects, early perturbations in blood flow can independently lead to critical heart defects. In this paper, we start by comparing the fetal circulation with the neonatal and adult circulation, and reviewing how altered fetal blood flow can be used as a diagnostic tool to plan interventions. We then look at known factors that lead to tetralogy of Fallot and persistent truncus arteriosus: namely early perturbations in blood flow and mutations within VEGF-related pathways. The interplay between physical and genetic factors means that any one alteration can cause significant disruptions during development and underscore our need to better understand the effects of both blood flow and flow-responsive genes. -

Genetic and Flow Anomalies in Congenital Heart Disease
Published online: 2021-05-10 AIMS Genetics, 3(3): 157-166. DOI: 10.3934/genet.2016.3.157 Received: 01 July 2016 Accepted: 16 August 2016 Published: 23 August 2016 http://www.aimspress.com/journal/Genetics Review Genetic and flow anomalies in congenital heart disease Sandra Rugonyi* Department of Biomedical Engineering, Oregon Health & Science University, 3303 SW Bond Ave. M/C CH13B, Portland, OR 97239, USA * Correspondence: Email: [email protected]; Tel: +1-503-418-9310; Fax: +1-503-418-9311. Abstract: Congenital heart defects are the most common malformations in humans, affecting approximately 1% of newborn babies. While genetic causes of congenital heart disease have been studied, only less than 20% of human cases are clearly linked to genetic anomalies. The cause for the majority of the cases remains unknown. Heart formation is a finely orchestrated developmental process and slight disruptions of it can lead to severe malformations. Dysregulation of developmental processes leading to heart malformations are caused by genetic anomalies but also environmental factors including blood flow. Intra-cardiac blood flow dynamics plays a significant role regulating heart development and perturbations of blood flow lead to congenital heart defects in animal models. Defects that result from hemodynamic alterations recapitulate those observed in human babies, even those due to genetic anomalies and toxic teratogen exposure. Because important cardiac developmental events, such as valve formation and septation, occur under blood flow conditions while the heart is pumping, blood flow regulation of cardiac formation might be a critical factor determining cardiac phenotype. The contribution of flow to cardiac phenotype, however, is frequently ignored. -

Prenatal Development Timeline
Prenatal Development Timeline Nervous Cardiovascular Muscular Early Events Special Senses Respiratory Skeletal Growth Parameters Blood & Immune Gastrointestinal Endocrine General Skin/Integument Renal/Urinary Reproductive Movement Unit 1: The First Week Day 0 — — Embryonic period begins Fertilization resulting in zygote formation Day 1 — — Embryo is spherically shaped with 12 to 16 cells Day 1 - Day 1 — — Fertilization - development begins with a single-cell embryo!!! Day 2 — — Early pregnancy factor (EPF) Activation of the genome Zygote divides into two blastomeres (24 – 30 hours from start of fertilization) Day 4 — — Embryonic disc Free floating blastocyst Hypoblast & epiblast Inner cell mass See where the back and chest will be Day 5 — — Hatching blastocyst Day 6 — — Embryo attaches to wall of uterus 1 week — — Chorion Placenta begins to form Unit 2: 1 to 2 Weeks 1 week, 1 day — — Amnioblasts present; amnion and amniotic cavity formation begins Positive pregnancy test 1 week, 2 days — — Cells in womb engorged with nutrients 1 week, 4 days — — Longitudinal axis 1 week, 5 days — — Implantation complete Yolk sac 1 week, 6 days — — Primordial blood vessels Amnion with single cell layer Chorionic villi 2 weeks — — Yolk sac Yolk sac Unit 3: 2 to 3 Weeks 2 weeks, 1 day — — 3 germ layers Rostral-caudal orientation 2 weeks, 2 days — — Erythroblasts in yolk sac Three types of blood-forming cells in yolk sac Amnion with two cell layers Secondary villi www.ehd.org 1 of 10 2 weeks, 4 days — — Foregut, midgut, and hindgut Brain is first organ to -

The Evolving Cardiac Lymphatic Vasculature in Development, Repair and Regeneration
REVIEWS The evolving cardiac lymphatic vasculature in development, repair and regeneration Konstantinos Klaourakis 1,2, Joaquim M. Vieira 1,2,3 ✉ and Paul R. Riley 1,2,3 ✉ Abstract | The lymphatic vasculature has an essential role in maintaining normal fluid balance in tissues and modulating the inflammatory response to injury or pathogens. Disruption of normal development or function of lymphatic vessels can have severe consequences. In the heart, reduced lymphatic function can lead to myocardial oedema and persistent inflammation. Macrophages, which are phagocytic cells of the innate immune system, contribute to cardiac development and to fibrotic repair and regeneration of cardiac tissue after myocardial infarction. In this Review, we discuss the cardiac lymphatic vasculature with a focus on developments over the past 5 years arising from the study of mammalian and zebrafish model organisms. In addition, we examine the interplay between the cardiac lymphatics and macrophages during fibrotic repair and regeneration after myocardial infarction. Finally, we discuss the therapeutic potential of targeting the cardiac lymphatic network to regulate immune cell content and alleviate inflammation in patients with ischaemic heart disease. The circulatory system of vertebrates is composed of two after MI. In this Review, we summarize the current complementary vasculatures, the blood and lymphatic knowledge on the development, structure and function vascular systems1. The blood vasculature is a closed sys- of the cardiac lymphatic vasculature, with an emphasis tem responsible for transporting gases, fluids, nutrients, on breakthroughs over the past 5 years in the study of metabolites and cells to the tissues2. This extravasation of cardiac lymphatic heterogeneity in mice and zebrafish. -

The Mechanisms of Hedgehog Signal Transduction
Markku Varjosalo The Mechanisms of Hedgehog Signal Transduction Publications of the National Public Health Institute A 12/2008 Department of Molecular Medicine National Public Health Institute and Institute of Biomedicine, University of Helsinki, Finland Helsinki, Finland 2008 Markku Varjosalo THE MECHANISMS OF HEDGEHOG SIGNAL TRANSDUCTION ACADEMIC DISSERTATION To be publicly discussed with the permission of the Faculty of Medicine, University of Helsinki, in Lecture Hall 2, Biomedicum Helsinki, on 2nd May 2008, at noon. National Public Health Institute, Helsinki, Finland and Institute of Biomedicine, University of Helsinki, Finland Helsinki 2008 Publications of the National Public Health Institute KTL A12 / 2008 Copyright National Public Health Institute Julkaisija-Utgivare-Publisher Kansanterveyslaitos (KTL) Mannerheimintie 166 00300 Helsinki Puh. vaihde (09) 474 41, telefax (09) 4744 8408 Folkhälsoinstitutet Mannerheimvägen 166 00300 Helsingfors Tel. växel (09) 474 41, telefax (09) 4744 8408 National Public Health Institute Mannerheimintie 166 FIN-00300 Helsinki, Finland Telephone +358 9 474 41, telefax +358 9 4744 8408 ISBN 978-951-740-805-9 ISSN 0359-3584 ISBN 978-951-740-806-6 (pdf) ISSN 1458-6290 (pdf) Kannen kuva - cover graphic: Yliopistopaino Helsinki 2008 Supervised by Academy Professor Jussi Taipale Genome-Scale Biology Program Institute of Biomedicine, University of Helsinki Department of Molecular Medicine National Public Health Institute (KTL) Helsinki, Finland Reviewed by Professor Tomi Mäkelä Genome-Scale Biology Program -

Dynamics of Maternal Morphogen Gradients in Drosophila Stanislav Y Shvartsman1, Mathieu Coppey1 and Alexander M Berezhkovskii2
COGEDE-512; NO OF PAGES 6 Available online at www.sciencedirect.com Dynamics of maternal morphogen gradients in Drosophila Stanislav Y Shvartsman1, Mathieu Coppey1 and Alexander M Berezhkovskii2 The first direct studies of morphogen gradients were done in ified genes [1]. Since most of these studies were done the end of 1980s, in the early Drosophila embryo, which is with fixed embryos, maternal gradients were viewed as patterned under the action of four maternally determined relatively static signals. Several recent studies report morphogens. Since the early studies of maternal morphogens quantitative measurements of the anterior, dorsoventral, were done with fixed embryos, they were viewed as relatively and terminal gradients and propose biophysical models static signals. Several recent studies analyze dynamics of the for their formation and interpretation. We review these anterior, dorsoventral, and terminal patterning signals. The studies and discuss how their conclusions affect our view results of these quantitative studies provide critical tests of of dynamics in one of the most extensively studied classical models and reveal new modes of morphogen patterning systems. regulation and readout in one of the most extensively studied patterning systems. Bicoid gradient: diffusion and reversible Addresses trapping of a stable protein? 1 Department of Chemical Engineering and Lewis-Sigler Institute for In the end of 1980s, studies of the distribution and Integrative Genomics, Princeton University, New Jersey, United States transcriptional effects of the Bicoid (Bcd) protein in 2 Mathematical and Statistical Computing Laboratory, Division of Computational Bioscience, Center for Information Technology, National the Drosophila embryo provided the first molecularly Institutes of Health, Bethesda, MD 20892, United States defined example of a morphogen gradient [2–4]. -

Differentiating Mesoderm and Muscle Cell Lineages During Drosophila Embryogenesis BRENDA LILLY, SAMUEL GALEWSKY, ANTHONY B
Proc. Nati. Acad. Sci. USA Vol. 91, pp. 5662-5666, June 1994 Developmental Biology D-MEF2: A MADS box transcription factor expressed in differentiating mesoderm and muscle cell lineages during Drosophila embryogenesis BRENDA LILLY, SAMUEL GALEWSKY, ANTHONY B. FIRULLI, ROBERT A. SCHULZ, AND ERIC N. OLSON* Department of Biochemistry and Molecular Biology, Box 117, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Boulevard, Houston, TX 77030 Communicated by Thomas P. Maniatis, January 21, 1994 ABSTRACT The myocyte enhancer factor (MEF) 2 family cle-specific genes (13). The recent cloning of MEF2 has oftranscription factors has been Implicated In the regultion of revealed that it belongs to the MADS (MCM1, Agamous, musle tansription in vertebrates. We have cloned a protein Deficiens, and serum-response factor) family oftranscription fromDrosophia, termed D-MEF2, that shares extensive amino factors. Four MEF2 genes, designated MEF2A, -B, -C, and acid homology with the MADS (MCM1, Agamous, Defdcens, -D, have been cloned from vertebrates (10, 14-19). The and serum-response factor) d of the vertebrate MEF2 proteins encoded by these genes are highly homologous proteins. D-mef2 gene expression Is first detected dring within the 55-amino-acid MADS domain at their amino Drosophila embryogenesis within mesodermal precursor cells termini and within an adjacent MEF2-specific region of 27 prior to spcation of the somatic and visceral muscle lin- residues, but they diverge outside of these regions. eages. Expression of D-mef2 Is dependent on the mesodermal We have cloned aDrosophila homologue ofMEF2, termed determinants twist and snail but independent ofthe homeobox- D-MEF2,t which, to our knowledge, is the first MADS containng gene dtnman, which is required for visceral muscle protein to be identified in Drosophila. -

Dynamics of the Dorsal Morphogen Gradient
Dynamics of the Dorsal morphogen gradient Jitendra S. Kanodiaa, Richa Rikhyb, Yoosik Kima, Viktor K. Lundc, Robert DeLottoc, Jennifer Lippincott-Schwartzb,1, and Stanislav Y. Shvartsmana,1 aDepartment of Chemical Engineering and Lewis-Sigler Institute for Integrative Genomics, Princeton University, Washington Road, Princeton, NJ 08544; bCell Biology and Metabolism Branch, NIH, Building 32, 18 Library Drive, Bethesda, MD 20892; and cDepartment of Molecular Biology, University of Copenhagen, Ole Maaløes Vej 5, DK-2200 Copenhagen, Denmark Contributed by Jennifer Lippincott-Schwartz, October 28, 2009 (sent for review September 6, 2009) The dorsoventral (DV) patterning of the Drosophila embryo depends several minutes (16). Nuclei change in volume and undergo five on the nuclear localization gradient of Dorsal (Dl), a protein related to synchronous divisions (15, 17). To explore how these processes the mammalian NF-B transcription factors. Current understanding of contribute to the formation of the Dl gradient, we formulated a how the Dl gradient works has been derived from studies of its mathematical model that accounts for the nuclear import and transcriptional interpretation, but the gradient itself has not been export of Dl, its interaction with Cact, and the dynamics of nuclear quantified. In particular, it is not known whether the Dl gradient is density and volumes in the syncytial blastoderm. Based on the stable or dynamic during the DV patterning of the embryo. To address computational analysis of this model and a number of our model- this question, we developed a mathematical model of the Dl gradient based experiments, we argue that the Dl gradient is dynamic and, and constrained its parameters by experimental data. -

The Physical Mechanisms of Drosophila Gastrulation: Mesoderm and Endoderm Invagination
| FLYBOOK DEVELOPMENT AND GROWTH The Physical Mechanisms of Drosophila Gastrulation: Mesoderm and Endoderm Invagination Adam C. Martin1 Department of Biology, Massachusetts Institute of Technology, Cambridge, Massachusetts 02142 ORCID ID: 0000-0001-8060-2607 (A.C.M.) ABSTRACT A critical juncture in early development is the partitioning of cells that will adopt different fates into three germ layers: the ectoderm, the mesoderm, and the endoderm. This step is achieved through the internalization of specified cells from the outermost surface layer, through a process called gastrulation. In Drosophila, gastrulation is achieved through cell shape changes (i.e., apical constriction) that change tissue curvature and lead to the folding of a surface epithelium. Folding of embryonic tissue results in mesoderm and endoderm invagination, not as individual cells, but as collective tissue units. The tractability of Drosophila as a model system is best exemplified by how much we know about Drosophila gastrulation, from the signals that pattern the embryo to the molecular components that generate force, and how these components are organized to promote cell and tissue shape changes. For mesoderm invagination, graded signaling by the morphogen, Spätzle, sets up a gradient in transcriptional activity that leads to the expression of a secreted ligand (Folded gastrulation) and a transmembrane protein (T48). Together with the GPCR Mist, which is expressed in the mesoderm, and the GPCR Smog, which is expressed uniformly, these signals activate heterotrimeric G-protein and small Rho-family G-protein signaling to promote apical contractility and changes in cell and tissue shape. A notable feature of this signaling pathway is its intricate organization in both space and time. -
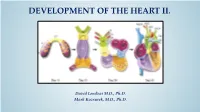
Development of Right Ventricle
DEVELOPMENT OF THE HEART II. David Lendvai M.D., Ph.D. Mark Kozsurek, M.D., Ph.D. • Septation of the common atrioventricular (AV) orifice. • Formation of the interatrial septum. • Formation of the muscular interventricular septum. • Appearance of the membranous interventricular septum and the spiral aorticopulmonary septum. right left septum primum septum primum septum primum septum primum septum primum septum primum foramen primum foramen primum septum primum septum primum foramen primum foramen primum septum primum septum primum foramen secundum foramen secundum foramen primum foramen primum septum primum foramen secundum septum primum foramen secundum foramen primum foramen primum septum primum septum primum foramen secundum foramen secundum septum secundum septum secundum foramen secundum foramen ovale foramen ovale septum primum septum primum septum secundum septum secundum foramen secundum foramen ovale foramen ovale septum primum septum primum septum secundum septum secundum foramen secundum septum primum foramen ovale foramen ovale septum primum SUMMARY • The septation of the common atrium starts with the appearance of the crescent-shaped septum primum. The opening of this septum, the foramen primum, becomes progressively smaller. • Before the foramen primum completly closes, postero-superiorly several small openings appear on the septum primum. These perforations coalesce later and form the foramen secundum. • On the right side of the septum primum a new septum, the septum secundum, starts to grow. The orifice of the septum secundum is the foramen ovale. • Finally two crescent-like, incomplete, partially overlapping septa exist with one hole on each. Septum secundum is more rigid and the septum primum on its left side acts as a valve letting the blood flow exclusively from the right to the left. -

Abnormalities of Placental Development and Function Are
bioRxiv preprint doi: https://doi.org/10.1101/388074; this version posted August 27, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abnormalities of placental development and function are associated with the different fetal growth patterns of hypoplastic left heart syndrome and transposition of the great arteries. Weston Troja1, Kathryn J. Owens1, Jennifer Courtney1, Andrea C. Hinton3, Robert B. Hinton3, James F. Cnota2 and Helen N. Jones1* 1. Center for Fetal and Placental Therapy, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, Cincinnati, Ohio 2. Heart Institute, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, Cincinnati, Ohio 3. TriHealth Maternal Fetal Medicine, 375 Dixsmyth Ave, Cincinnati Ohio *Corresponding Author: Helen N. Jones [email protected] 1 bioRxiv preprint doi: https://doi.org/10.1101/388074; this version posted August 27, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Background: Birthweight is a critical predictor of congenital heart disease (CHD) surgical outcomes. Hypoplastic left heart syndrome (HLHS) is cyanotic CHD with known fetal growth restriction and placental abnormalities. Transposition of the great arteries (TGA) is cyanotic CHD with normal fetal growth. Comparison of the placenta in these diagnoses may provide insights on the fetal growth abnormality of CHD. Methods: Clinical data and placental histology from placentas associated with Transposition of the Great Arteries (TGA) were analyzed for gross pathology, morphology, maturity and vascularity and compared to both control and previously analyzed HLHS placentas [1].