Vertebrate Embryos As Tools for Anti-Angiogenic Drug Screening and Function
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cardiogenesis with a Focus on Vasculogenesis and Angiogenesis
Received: 27 August 2019 | Revised: 4 February 2020 | Accepted: 20 February 2020 DOI: 10.1111/ahe.12549 SPECIAL ISSUE Cardiogenesis with a focus on vasculogenesis and angiogenesis Katrin Borasch1 | Kenneth Richardson2 | Johanna Plendl1 1Department of Veterinary Medicine, Institute of Veterinary Anatomy, Freie Abstract University Berlin, Berlin, Germany The initial intraembryonic vasculogenesis occurs in the cardiogenic mesoderm. Here, 2 College of Veterinary Medicine, School a cell population of proendocardial cells detaches from the mesoderm that subse- of Veterinary and Life Sciences, Murdoch University, Murdoch, WA, Australia quently generates the single endocardial tube by forming vascular plexuses. In the course of embryogenesis, the endocardium retains vasculogenic, angiogenic and Correspondence Johanna Plendl, Department of Veterinary haematopoietic potential. The coronary blood vessels that sustain the rapidly ex- Medicine, Institute of Veterinary Anatomy, panding myocardium develop in the course of the formation of the cardiac loop by Freie University Berlin, Berlin, Germany. Email: [email protected] vasculogenesis and angiogenesis from progenitor cells of the proepicardial serosa at the venous pole of the heart as well as from the endocardium and endothelial cells of Funding information Freie Universität Berlin the sinus venosus. Prospective coronary endothelial cells and progenitor cells of the coronary blood vessel walls (smooth muscle cells, perivascular cells) originate from different cell populations that are in close spatial as well as regulatory connection with each other. Vasculo- and angiogenesis of the coronary blood vessels are for a large part regulated by the epicardium and epicardium-derived cells. Vasculogenic and angiogenic signalling pathways include the vascular endothelial growth factors, the angiopoietins and the fibroblast growth factors and their receptors. -

Cardiovascular System Heart Development Cardiovascular System Heart Development
Cardiovascular System Heart Development Cardiovascular System Heart Development In human embryos, the heart begins to beat at approximately 22-23 days, with blood flow beginning in the 4th week. The heart is one of the earliest differentiating and functioning organs. • This emphasizes the critical nature of the heart in distributing blood through the vessels and the vital exchange of nutrients, oxygen, and wastes between the developing baby and the mother. • Therefore, the first system that completes its development in the embryo is called cardiovascular system. https://www.slideshare.net/DrSherifFahmy/intraembryonic-mesoderm-general-embryology Mesoderm is one of the three • Connective tissue primary germ layers that • Smooth and striated muscle • Cardiovascular System differentiates early in • Kidneys development that collectively • Spleen • Genital organs, ducts gives rise to all subsequent • Adrenal gland cortex tissues and organs. The cardiovascular system begins to develop in the third week of gestation. Blood islands develop in the newly formed mesoderm, and consist of (a) a central group of haemoblasts, the embryonic precursors of blood cells; (b) endothelial cells. Development of the heart and vascular system is often described together as the cardiovascular system. Development begins very early in mesoderm both within (embryonic) and outside (extra embryonic, vitelline, umblical and placental) the embryo. Vascular development occurs in many places. • Blood islands coalesce to form a vascular plexus. Preferential channels form arteries and veins. • Day 17 - Blood islands form first in the extra-embryonic mesoderm • Day 18 - Blood islands form next in the intra-embryonic mesoderm • Day 19 - Blood islands form in the cardiogenic mesoderm and coalesce to form a pair of endothelial heart tubes Development of a circulation • A circulation is established during the 4th week after the myocardium is differentiated. -

Cardiovascular System Note: the Cardiovascular System Develops Early (Week 3), Enabling the Embryo to Grow Beyond the Short
Lymphatics: Lymph vessel formation is similar to blood angiogenesis. Lymphatics begin as lymph sacs in three regions: jugular (near brachiocephalic veins); cranial abdominal (future cysterna chyla); and iliac region. Lym- phatic vessels (ducts) form as outgrowths of the sacs. mesenchyme Lymph nodes are produced by localized mesoder- sinusoid lymph duct lumen mal invaginations that partition the vessel lumen into sinu- soids. The mesoderm develops a reticular framework within which mesodermal lymphocytes accumulate. The spleen and hemal nodes (in ruminants) invagination develop similar to the way lymph nodes develop. Lymph Node Formation Prior to birth, fetal circulation is designed for an in utero aqueous environment where the pla- centa oxygenates fetal blood. Suddenly, at birth... Three In-Utero Adjustments ductus Stretching and constriction of arteriosus umbilical arteries shifts fetal blood flow aortic arch from the placenta to the fetus. Reduced pulmonary trunk L atrium venous return through the (left) umbili- foramen ovale R cal vein and ductus venosus allows the atrium latter to gradually close (over a period caudal vena cava of days). Bradykinin released by expand- ductus venosus ing lungs and increased oxygen concen- tration in blood triggers constriction of aorta the ductus arteriosus which, over two liver months, is gradually converted to a fibrous structure, the ligamentum arte- umbilical v. riosum. portal v. The increased blood flow to the lungs and then to the left atrium equalizes pres- sure in the two atria, resulting in closure umbilical aa. of the foramen ovale that eventually grows permanent. 29 The cardiogenic area, the place where the embryonic heart originates, is located . -

The Functional Anatomy of the Heart. Development of the Heart, Anomalies
The functional anatomy of the heart. Development of the heart, anomalies Anatomy and Clinical Anatomy Department Anastasia Bendelic Plan: Cardiovascular system – general information Heart – functional anatomy Development of the heart Abnormalities of the heart Examination in a living person Cardiovascular system Cardiovascular system (also known as vascular system, or circulatory system) consists of: 1. heart; 2. blood vessels (arteries, veins, capillaries); 3. lymphatic vessels. Blood vessels Arteries are blood vessels that carry blood away from the heart. Veins carry blood back towards the heart. Capillaries are tiny blood vessels, that connect arteries to veins. Lymphatic vessels: lymphatic capillaries; lymphatic vessels (superficial and deep lymph vessels); lymphatic trunks (jugular, subclavian, bronchomediastinal, lumbar, intestinal trunks); lymphatic ducts (thoracic duct and right lymphatic duct). Lymphatic vessels Microcirculation Microcirculatory bed comprises 7 components: 1. arterioles; 2. precapillaries or precapillary arterioles; 3. capillaries; 4. postcapillaries or postcapillary venules; 5. venules; 6. lymphatic capillaries; 7. interstitial component. Microcirculation The heart Heart is shaped as a pyramid with: an apex (directed downward, forward and to the left); a base (facing upward, backward and to the right). There are four surfaces of the heart: sternocostal (anterior) surface; diaphragmatic (inferior) surface; right pulmonary surface; left pulmonary surface. External surface of the heart The heart The heart has four chambers: right and left atria; right and left ventricles. Externally, the atria are demarcated from the ventricles by coronary sulcus (L. sulcus coronarius). The right and left ventricles are demarcated from each other by anterior and posterior interventricular sulci (L. sulci interventriculares anterior et posterior). Chambers of the heart The atria The atria are thin-walled chambers, that receive blood from the veins and pump it into the ventricles. -
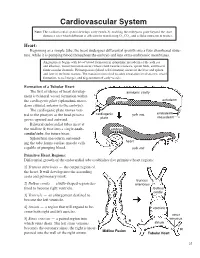
Cardiovascular System Note: the Cardiovascular System Develops Early (Week-3), Enabling the Embryo to Grow Beyond the Short
Cardiovascular System Note: The cardiovascular system develops early (week-3), enabling the embryo to grow beyond the short distances over which diffusion is efficient for transferring 2O , CO2, and cellular nutrients & wastes. Heart: Beginning as a simple tube, the heart undergoes differential growth into a four chambered struc- ture, while it is pumping blood throughout the embryo and into extra-embryonic membranes. Angiogenesis begins with blood island formation in splanchnic mesoderm of the yolk sac and allantois. Vessel formation occurs when island vesicles coalesce, sprout buds, and fuse to form vascular channels. Hematopoiesis (blood cell formation) occurs in the liver and spleen and later in the bone marrow. The transition from fetal to adult circulation involves new vessel formation, vessel merger, and degeneration of early vessels. Formation of a Tubular Heart: The first evidence of heart develop- amnionic cavity ment is bilateral vessel formation within ectoderm the cardiogenic plate (splanchnic meso- embryo derm situated anterior to the embryo). The cardiogenic plate moves ven- tral to the pharynx as the head process cardiogenic yolk sac endoderm mesoderm grows upward and outward. plate Bilateral endocardial tubes meet at the midline & fuse into a single endo- embryo cardial tube, the future heart. Splanchnic mesoderm surround- ing the tube forms cardiac muscle cells heart capable of pumping blood. yolk sac Primitive Heart Regions: Differential growth of the endocardial tube establishes five primitive heart regions: 1] Truncus arteriosus — the output region of the heart. It will develop into the ascending aorta and pulmonary trunk. truncus 2] Bulbus cordis — a bulb-shaped region des- arteriosus tined to become right ventricle. -

Functional Morphology of the Cardiac Jelly in the Tubular Heart of Vertebrate Embryos
Review Functional Morphology of the Cardiac Jelly in the Tubular Heart of Vertebrate Embryos Jörg Männer 1,*,† and Talat Mesud Yelbuz 2,† 1 Group Cardio‐Embryology, Institute of Anatomy and Embryology UMG, Georg‐August‐University Goettingen, D‐37075 Goettingen, Germany; [email protected] 2 Department of Cardiac Sciences, King Abdulaziz Cardiac Center, Section of Pediatric Cardiology, King Abdulaziz Medical City, Ministry of National Guard Health Affairs, Riyadh 11426, Saudi Arabia; [email protected] * Correspondence: [email protected]; Tel.: +49‐551‐39‐7032 † This work is dedicated to the memory of our academic mentors Gerd Steding (1936–2011) and Armin Wessel (1946–2011). Received: 29 January 2019; Accepted: 21 February 2019; Published: 27 February 2019 Abstract: The early embryonic heart is a multi‐layered tube consisting of (1) an outer myocardial tube; (2) an inner endocardial tube; and (3) an extracellular matrix layer interposed between the myocardium and endocardium, called “cardiac jelly” (CJ). During the past decades, research on CJ has mainly focused on its molecular and cellular biological aspects. This review focuses on the morphological and biomechanical aspects of CJ. Special attention is given to (1) the spatial distribution and fiber architecture of CJ; (2) the morphological dynamics of CJ during the cardiac cycle; and (3) the removal/remodeling of CJ during advanced heart looping stages, which leads to the formation of ventricular trabeculations and endocardial cushions. CJ acts as a hydraulic skeleton, displaying striking structural and functional similarities with the mesoglea of jellyfish. CJ not only represents a filler substance, facilitating end‐systolic occlusion of the embryonic heart lumen. -

Cardiogenesis in the Bovine to 35 Somites
CARDIOGENESIS IN THE BOVINE TO 35 SOMITES by PATRICIA ANN NODEN A. A., Chanute Junior College, 1962 B. S., Kansas State University, 1964 A MASTER'S THESIS submitted in partial fulfillment of the requirements for the degree MASTER OF SCIENCE Department of Zoology KANSAS STATE UNIVERSITY Manhattan, Kansas 1966 Approved by: ii N7G> TABLE OF CONTENTS Oocu mtA t INTRODUCTION 1 METHODS AND MATERIALS 6 OBSERVATIONS 9 Presomite Stage 9 Six Somite Stage 9 Nine Somite Stage 10 13 Somite Stage 18 19 Somite Stage 18 22 Somite Stage 19 24-25 Somite Stage 20 28-30 Somite Stage ....... 21 33-35 Somite Stage 24 DISCUSSION 27 CONCLUSION S3 ACKNOWLEDGMENTS 35 BIBLIOGRAPHY . 36 . INTRODUCTION Mammalian cardiogenesis is a vast field which so far has not been thoroughly explored. There are few species in which the complete development of the heart has been studied and many in which partial formation has been observed The formation of the heart to the functional stage in the dog (14 somites) was studied by Bonnet (1901) and Duffey (1953). Duffey's thesis was used exclusively for comparison in this paper. The rat has been studied in some detail, from presomite to birth and after, by Burlingame and Long (1939) and early stages by Ravn (1895) and Goss (1942, 1952). The tubular phase of cardiogenesis in the rabbit of 10-13 somites was described by Girgis (1930, 1933). Dwinnel (1939) worked with early somite stages. Development of the aortic arches was described by Bremer (1912). Hensen (1875), Bom (1888, 1889), Strahl and Carius (1889) and Rouviere (1904) have described early cardiac formation. -

Functional Morphology of the Cardiac Jelly in the Tubular
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 30 January 2019 doi:10.20944/preprints201901.0312.v1 Peer-reviewed version available at J. Cardiovasc. Dev. Dis. 2019, 6, 12; doi:10.3390/jcdd6010012 1 Review 2 Functional Morphology of the Cardiac Jelly in the 3 Tubular Heart of Vertebrate Embryos 4 Jörg Männer 1,* and Talat Mesud Yelbuz 2 5 1 Group Cardio-Embryology, Institute of Anatomy and Embryology UMG, Georg-August-University 6 Goettingen, D-37075 Goettingen, Germany; [email protected] 7 2 Department of Cardiac Sciences, King Abdulaziz Cardiac Center, Section of Pediatric Cardiology, King 8 Abdulaziz Medical City, Ministry of National Guard Health Affairs; Riyadh, Kingdom of Saudi Arabia; 9 [email protected] 10 * Correspondence: [email protected]; Tel.: +49-551-39-7032 11 12 Abstract: The early embryonic heart is a multi-layered tube consisting of (1) an 13 outer myocardial tube; (2) an inner endocardial tube; and (3) an extracellular 14 matrix layer interposed between myocardium and endocardium, called “cardiac 15 jelly” (CJ). During the past decades, research on CJ has mainly focused on its 16 molecular and cell biological aspects. This review focuses on the morphological 17 and biomechanical aspects of CJ. Special attention is given to (1) the spatial 18 distribution and fiber architecture of CJ; (2) the morphological dynamics of CJ 19 during the cardiac cycle; and (3) the removal/remodeling of CJ during advanced 20 heart looping stages, which leads to the formation of ventricular trabeculations 21 and endocardial cushions. CJ acts as a hydraulic skeleton displaying striking 22 structural and functional similarities with the mesoglea of jellyfish. -

Cardiac Development
2 Cardiac Development BRAD J. MARTINSEN, PhD AND JAMIE L. LOHR, MD CONTENTS INTRODUCTION TO HUMAN HEART EMBRYOLOGY AND DEVELOPMENT PRIMARY HEART FIELD AND LINEAR HEART TUBE FORMATION SECONDARY HEART FIELD, OUTFLOWTRACT FORMATION, AND CARDIAC LOOPING CARDIAC NEURAL CREST AND OUTFLOWTRACT AND ATRIAL AND VENTRICULARSEPTATION PROEPICARDIUM AND CORONARY ARTERY DEVELOPMENT CARDIAC MATURATION SUMMARY OF EMBRYONIC CONTRIBUTIONTO HEART DEVELOPMENT REFERENCES 1. INTRODUCTION TO HUMAN HEART human congenital heart disease (5). In addition, great strides EMBRYOLOGY AND DEVELOPMENT have also been made in our knowledge of the contribution of the cardiac neural crest and the epicardium to overall heart devel- The primary heart field, secondary heart field, cardiac neural opment. crest, and proepicardium are the four major embryonic regions involved in the process of vertebrate heart develop- 2. PRIMARY HEART FIELD ment (Fig. 1). They each make an important contribution to AND LINEAR HEART TUBE FORMATION overall cardiac development, which occurs with complex developmental timing and regulation. This chapter describes The cells that will become the heart are among the first cell how these regions interact to form the final structure of the heart lineages formed in the vertebrate embryo (6,7). By day 15 of in relationship to the generalized developmental timeline of human development, the primitive streak has formed (8), and human embryology (Table 1). the first mesodermal cells to migrate (gastrulate) through the The heart is the first organ to fully form and function during primitive streak are cells fated to become the heart (9,10) vertebrate development, and many of the underlying mecha- (Fig. 2). These mesodermal cells migrate to an anterior and nisms are considered molecularly and developmentally con- lateral position where they form bilateral primary heart fields served (1). -

6. Heart and Circulatory System I
6. HEART AND CIRCULATORY SYSTEM I Dr. Taube P. Rothman P&S 12-520 [email protected] 212-305-7930 RECOMMENDED READING: Larsen Human Embryology, 3rd Edition, pp. 195-199; 157-169 top left; 172-174; bottom 181-182; 187-top 189, Simbryo-cardiovascular system SUMMARY: The circulatory system, consisting of heart, blood vessels, and blood cells is the first functional organ to develop. This lecture will focus on the formation of the embryonic vasculature, the origin and formation of the early heart tube and primitive cardiac chambers, cardiac looping, and the primitive circulation. Between the 5th - 8th week of embryonic development, the tubular heart is remodeled into a four chambered structure. We will see how right and left atrioventricular canals connect each atrium with its respective ventricle, and how the atrial septum and definitive right and left atria form. We will also see why the great veins deliver blood to the right atrium while the pulmonary veins empty into the left. GLOSSARY: Angioblasts: precursors of blood vessels Angiogenesis: lengthening, branching, sprouting and remodeling of embryonic blood vessels Aortic arches: paired arteries surrounding the pharynx; portions will contribute to formation of the great arterial vessels Blood islands: clusters of cells in the yolk sac, connecting stalk and chorionic villi that form primitive blood vessels Cardiac jelly: gelatinous extracellular matrix that forms the middle layer of the heart tube Ductus venosus: shunts most of the blood in the umbilical vein into the inferior vena cava -

Atrioventricular Node (AVN) Originates from (I) the Left Wall of Sinus Venosus and (Ii) Superior Endocardial Cushion of the Atriventricular Canal
W.S. O School of Biomedical Sciences, University of Hong Kong Objectives: • Describe early angiogenesis. • Describe the heart tube formation. • Describe the partitioning into a 4- chambered heart. • List the formation of heart valves and the conducting system in the heart. • Explain the basis of congenital heart malformation. Day 25 Day 20 Heart tubes and Intraembryonic embryonic vessels circulation established develop 6 weeks 4-5 weeks Heart tube partitions into 4 Aortic arches heart chambers transform 8 weeks Heart valves Birth completed Foramen ovale closes with increased blood flow from lungs Blood and blood vessels Primitive blood cells Angioblast (mesodermal) Endothelial cells Connective tissue surrounding mesoderm Smooth muscles Blood vessels formation The three parts of circulation: vitelline, chorionic & intraembryonic Molecular regulation of cardiac development • Heart induction – BMPs secreted by endoderm and lateral plate mesoderm together with inhibition of WNT in the anterior part of the embryo induce the expression of NKX-2.5 in the heart -forming region . • Cardiac looping - is induced by the nodal and lefty2 genes. These two genes induce the transcription factor of PITX2 in deposition of extracellular matrix in looping. Development of the heart - 1 1. Cardiogenic plate appears around 3rd week Anterior to prochordal plate Pericardial coelom develop around heart tube 2. Rotation of heart primordia –180 rotation 3. Fusion of heart tubes around 4th week; 4. Differentiation of the heart tube tissue Epicardium – connective tissue Myocardium – cardiac muscle Subendocardium – spongy reticular tissue Endocardium - endothelium Embryonic development of the heart. (a) Endocardial heart tubes start to fuse, (b) Fusion of heart tubes complete, (c) Division of heart tube into dilated segments, (d) Looping of the heart tube, (e) Looping complete, (f) Frontal section of heart tube. -

Development of CVS I
Development of CVS I Systemic Embryology Heart development (cardiogenesis) • Prenatal development of the human heart • Begins with the formation of two endocardial tubes • These tubes merge to form the tubular heart, also called the primitive heart tube • Primitive heart tube loops and septates into four chambers and paired arterial trunks that form the adult heart. 2 3 Early development of the heart 4 • First major system to function in the embryo. • Primordial heart and vascular system appear in the middle of the third week (Day 21or 22). - The rapidly growing embryo can no longer satisfy its nutritional and oxygen requirements by diffusion alone. - A need for an efficient method of acquiring oxygen and nutrients from the maternal blood and disposing of carbon dioxide and waste products. 5 Derived from: • Splanchnic mesoderm, which forms the primordium of the heart • Paraxial and lateral mesoderm • Pharyngeal mesoderm • Neural crest cells 6 • In the splanchnopleuric mesenchyme on either side of the neural plate, a horseshoe-shaped area develops as the cardiogenic region • Formed from cardiac myoblasts and blood islands • By day 19, an endocardial tube begins to develop in each side of this region • Tubes grow, converge and merge to form a single tube – the tubular heart by programmed cell death 7 • Cardiac progenitor cells in the epiblast, lateral to the primitive streak migrate through the streak. • Cardiogenic region develops cranially and laterally to the neural plate • As embryonic folding continues, the two endocardial tubes are pushed into the thoracic cavity, where they fuse together at about the 22nd day 8 • Heart develop near the head of the embryo in the cardiogenic region • Cells in the splanchnic layer of the lateral plate mesoderm are induced by pharyngeal endoderm to form cardiac myoblasts.