In Comparison to Two Common Locations for Aventricular Septal Defect (Right), the Most Common Form of Congenital Heart Defect
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Usefulness Ofcontinuous Positive Airway Pressure in Differential
Arch Dis Child: first published as 10.1136/adc.53.6.456 on 1 June 1978. Downloaded from Archives of Disease in Childhood, 1978, 53, 456-460 Usefulness of continuous positive airway pressure in differential diagnosis of cardiac from pulmonary cyanosis in newborn infants P. SYAMASUNDAR RAO, BRENDA L. MARINO, AND ALEX F. ROBERTSON III From the Department of Pediatrics, Sections of Pediatric Cardiology and Neonatology, Medical College of Georgia, Augusta, Georgia, USA SUMMARY Differential diagnosis of cyanosis in the neonate is difficult and cardiac catheterisa- tion may be required for a correct diagnosis. It has been suggested that the response of Pao2 to continuous positive airway pressure (CPAP) with 100% oxygen may be useful. The purpose of this study was to test further this hypothesis by studying all neonates investigated for cyanosis with a Pao2 -50 torr in 0 8 to 1 .0 F1o2. Arterial blood samples were obtained in an F1o2 of 0 21-0 .4 and 0 8-1 .0, and in an F1O2 of 0 8-1 0 with 8-10 cm CPAP, and were analysed for Pao2, Paco2, and pH, bicarbonate being calculated. The final diagnoses were congenital heart disease (CHD) 21 cases, pulmonary parenchymal disease (PD) 10 cases, and persistent fetal circulation (PFC) 3 cases. No significant difference in pH, bicarbonate, or Paco2 was observed among the three groups or with CPAP. In the CHD and PFC infants CPAP produced no significant change in Pao2. In the PD babies Pao2 increased by an average of 33 torr (P<0 05). Despite thus attaining statistical significance 2 PD infants had no increase in Pao2 with CPAP. -

Pharmacy Policy Statement
PHARMACY POLICY STATEMENT Ohio Medicaid DRUG NAME Synagis (palivizumab) BILLING CODE 90378 (1 unit = 1 vial) BENEFIT TYPE Medical SITE OF SERVICE ALLOWED Office/Outpatient Hospital/Home COVERAGE REQUIREMENTS Prior Authorization Required (Preferred Product) QUANTITY LIMIT— 1 vial per month (max 5 during respiratory syncytial virus season) LIST OF DIAGNOSES CONSIDERED NOT Click Here MEDICALLY NECESSARY Synagis (palivizumab) is a preferred product and will only be considered for coverage under the medical/pharmacy benefit when the following criteria are met: Members must be clinically diagnosed with one of the following disease states and meet their individual criteria as stated. PREVENTION OF RESPIRATORY TRACT DISEASE CAUSED BY RESPIRATORY SYNCYTIAL VIRUS (RSV) For initial authorization: 1. Request must be made during the RSV season (November 1st through March 31st) AND initiation of injections should be timed with the onset of laboratory confirmed cases of RSV activity in the community, no earlier than November 1, 2017; AND 2. Member is < 12 months old at the beginning of the RSV season AND meet one of the following criteria (chart notes must be provided to support evidence): a) Member was born < 29 weeks, 0 days’ gestation; b) Member has Chronic Lung Disease (CLD) of prematurity (defined as gestational age <32 weeks, 0 days and a requirement for >21% oxygen for at least the first 28 days after birth); c) Member has hemodynamically significant Congenital Heart Disease (CHD) with one or more of the following: i) Acyanotic heart disease (e.g. atrial septal defect (ASD), ventricular septal defect (VSD), patent ductus arteriosus (PDA), etc.), AND member is receiving medication to control congestive heart failure (CHF) AND will require cardiac surgical procedures; ii) Moderate to severe pulmonary hypertension; iii) Cyanotic heart defect (e.g. -

Cardiogenesis with a Focus on Vasculogenesis and Angiogenesis
Received: 27 August 2019 | Revised: 4 February 2020 | Accepted: 20 February 2020 DOI: 10.1111/ahe.12549 SPECIAL ISSUE Cardiogenesis with a focus on vasculogenesis and angiogenesis Katrin Borasch1 | Kenneth Richardson2 | Johanna Plendl1 1Department of Veterinary Medicine, Institute of Veterinary Anatomy, Freie Abstract University Berlin, Berlin, Germany The initial intraembryonic vasculogenesis occurs in the cardiogenic mesoderm. Here, 2 College of Veterinary Medicine, School a cell population of proendocardial cells detaches from the mesoderm that subse- of Veterinary and Life Sciences, Murdoch University, Murdoch, WA, Australia quently generates the single endocardial tube by forming vascular plexuses. In the course of embryogenesis, the endocardium retains vasculogenic, angiogenic and Correspondence Johanna Plendl, Department of Veterinary haematopoietic potential. The coronary blood vessels that sustain the rapidly ex- Medicine, Institute of Veterinary Anatomy, panding myocardium develop in the course of the formation of the cardiac loop by Freie University Berlin, Berlin, Germany. Email: [email protected] vasculogenesis and angiogenesis from progenitor cells of the proepicardial serosa at the venous pole of the heart as well as from the endocardium and endothelial cells of Funding information Freie Universität Berlin the sinus venosus. Prospective coronary endothelial cells and progenitor cells of the coronary blood vessel walls (smooth muscle cells, perivascular cells) originate from different cell populations that are in close spatial as well as regulatory connection with each other. Vasculo- and angiogenesis of the coronary blood vessels are for a large part regulated by the epicardium and epicardium-derived cells. Vasculogenic and angiogenic signalling pathways include the vascular endothelial growth factors, the angiopoietins and the fibroblast growth factors and their receptors. -

Vertebrate Embryos As Tools for Anti-Angiogenic Drug Screening and Function
Vertebrate embryos as tools for anti-angiogenic drug screening and function Shaunna Beedie1,2, Alexandra J. Diamond1, Lucas Rosa Fraga1, William D. Figg2, Neil Vargesson1, * 1 School of Medicine, Medical Sciences and Nutrition, Institute of Medical Sciences, University of Aberdeen, Foresterhill, Aberdeen, UK. 2 Molecular Pharmacology Section, Genitourinary Malignancies Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health. Bethesda. USA. *Corresponding author Neil Vargesson: [email protected]; [email protected] Key words: angiogenesis, chicken, zebrafish, mouse, rat, rabbit, non-human primates, thalidomide Abstract The development of new angiogenic inhibitors highlights a need for robust screening assays that adequately capture the complexity of vessel formation, and allow for the quantitative evaluation of the teratogenicity of new anti- angiogenic agents. This review discusses the use of screening assays in vertebrate embryos, specifically focusing upon chicken and zebrafish embryos, for the detection of anti-angiogenic agents. Introduction and background The cardiovascular system is vital for normal embryonic development in utero [1]. It is one of the earliest differentiating and functioning organ systems, emphasizing its importance to the embryo [2-7]. The primitive vascular system develops by vasculogenesis, de novo differentiation and growth of vessels from the mesoderm. The major vessels in the embryo form first, the dorsal aortae (transporting oxygenated blood from the placenta or yolk sac to the heart) and vena cava or vitelline veins (transporting deoxygenated blood back to the heart or yolk sac) develop by vasculogenesis. Expansion of the nascent vascular network can then occur by angiogenesis, the process of vessel formation from the preexisting vasculature. -

Cardiovascular System Heart Development Cardiovascular System Heart Development
Cardiovascular System Heart Development Cardiovascular System Heart Development In human embryos, the heart begins to beat at approximately 22-23 days, with blood flow beginning in the 4th week. The heart is one of the earliest differentiating and functioning organs. • This emphasizes the critical nature of the heart in distributing blood through the vessels and the vital exchange of nutrients, oxygen, and wastes between the developing baby and the mother. • Therefore, the first system that completes its development in the embryo is called cardiovascular system. https://www.slideshare.net/DrSherifFahmy/intraembryonic-mesoderm-general-embryology Mesoderm is one of the three • Connective tissue primary germ layers that • Smooth and striated muscle • Cardiovascular System differentiates early in • Kidneys development that collectively • Spleen • Genital organs, ducts gives rise to all subsequent • Adrenal gland cortex tissues and organs. The cardiovascular system begins to develop in the third week of gestation. Blood islands develop in the newly formed mesoderm, and consist of (a) a central group of haemoblasts, the embryonic precursors of blood cells; (b) endothelial cells. Development of the heart and vascular system is often described together as the cardiovascular system. Development begins very early in mesoderm both within (embryonic) and outside (extra embryonic, vitelline, umblical and placental) the embryo. Vascular development occurs in many places. • Blood islands coalesce to form a vascular plexus. Preferential channels form arteries and veins. • Day 17 - Blood islands form first in the extra-embryonic mesoderm • Day 18 - Blood islands form next in the intra-embryonic mesoderm • Day 19 - Blood islands form in the cardiogenic mesoderm and coalesce to form a pair of endothelial heart tubes Development of a circulation • A circulation is established during the 4th week after the myocardium is differentiated. -

Cardiovascular System Note: the Cardiovascular System Develops Early (Week 3), Enabling the Embryo to Grow Beyond the Short
Lymphatics: Lymph vessel formation is similar to blood angiogenesis. Lymphatics begin as lymph sacs in three regions: jugular (near brachiocephalic veins); cranial abdominal (future cysterna chyla); and iliac region. Lym- phatic vessels (ducts) form as outgrowths of the sacs. mesenchyme Lymph nodes are produced by localized mesoder- sinusoid lymph duct lumen mal invaginations that partition the vessel lumen into sinu- soids. The mesoderm develops a reticular framework within which mesodermal lymphocytes accumulate. The spleen and hemal nodes (in ruminants) invagination develop similar to the way lymph nodes develop. Lymph Node Formation Prior to birth, fetal circulation is designed for an in utero aqueous environment where the pla- centa oxygenates fetal blood. Suddenly, at birth... Three In-Utero Adjustments ductus Stretching and constriction of arteriosus umbilical arteries shifts fetal blood flow aortic arch from the placenta to the fetus. Reduced pulmonary trunk L atrium venous return through the (left) umbili- foramen ovale R cal vein and ductus venosus allows the atrium latter to gradually close (over a period caudal vena cava of days). Bradykinin released by expand- ductus venosus ing lungs and increased oxygen concen- tration in blood triggers constriction of aorta the ductus arteriosus which, over two liver months, is gradually converted to a fibrous structure, the ligamentum arte- umbilical v. riosum. portal v. The increased blood flow to the lungs and then to the left atrium equalizes pres- sure in the two atria, resulting in closure umbilical aa. of the foramen ovale that eventually grows permanent. 29 The cardiogenic area, the place where the embryonic heart originates, is located . -

Review Article Congenital Heart Diseases
KYAMC Journal Vol. 9, No.1, April 2018 Review Article Congenital heart diseases: A review of echocardiogram records Md. Saiful Islam1, Md. Moniruzzaman2 Abstract Congenital heart defect (CHD) means an anatomic malformation of the heart or great vessels which occurs during intrauterine development, irrespective of the age at presentation. They can disrupt the normal blood flow through the heart. The blood flow can slow down, go in the wrong direction or to the wrong place, or be blocked completely. Broadly congenital heart defects can be acyanotic and cyanotic. We have reviewed retrospectively from echocardiogram record nearly two years of period & collected total 404 patients with congenital heart defects. Among them 329 (81.43%) was acyanotic and 75 (18.57%) was cyanotic congenital defects with variety of diagnosis. Ventricular septal defect was the most common acyanotic heart defect and Tetralogy of Fallot was the most common cyanotic heart defect. There was no significant gender deference. Keywords: Acyanotic, Congenital heart disease, Cyanotic. Date of received: 11. 11. 2017 Date of acceptance: 05. 01. 2018 Introduction known. The majority of the defects can be explained by Congenital heart defects (CHD) are reported in almost 1% of multifactorial inheritance hypothesis which states that a live births, and about half of these children need medical or predisposed fetus, when exposed to a given environmental surgical management in infancy1. In the first decade, a further trigger, to which the fetus is sensitive during the critical period 25% require surgery to maintain or improve their life1. Only of cardiac morphogenesis may develop the disease5. A variety 10% survive to adolescence without specific treatment. -

Pulmonary-Atresia-Mapcas-Pavsdmapcas.Pdf
Normal Heart © 2012 The Children’s Heart Clinic NOTES: Children’s Heart Clinic, P.A., 2530 Chicago Avenue S, Ste 500, Minneapolis, MN 55404 West Metro: 612-813-8800 * East Metro: 651-220-8800 * Toll Free: 1-800-938-0301 * Fax: 612-813-8825 Children’s Minnesota, 2525 Chicago Avenue S, Minneapolis, MN 55404 West Metro: 612-813-6000 * East Metro: 651-220-6000 © 2012 The Children’s Heart Clinic Reviewed March 2019 Pulmonary Atresia, Ventricular Septal Defect and Major Aortopulmonary Collateral Arteries (PA/VSD/MAPCAs) Pulmonary atresia (PA), ventricular septal defect (VSD) and major aortopulmonary collateral arteries (MAPCAs) is a rare type of congenital heart defect, also referred to as Tetralogy of Fallot with PA/MAPCAs. Tetralogy of Fallot (TOF) is the most common cyanotic heart defect and occurs in 5-10% of all children with congenital heart disease. The classic description of TOF includes four cardiac abnormalities: overriding aorta, right ventricular hypertrophy (RVH), large perimembranous ventricular septal defect (VSD), and right ventricular outflow tract obstruction (RVOTO). About 20% of patients with TOF have PA at the infundibular or valvar level, which results in severe right ventricular outflow tract obstruction. PA means that the pulmonary valve is closed and not developed. When PA occurs, blood can not flow through the pulmonary arteries to the lungs. Instead, the child is dependent on a patent ductus arteriosus (PDA) or multiple systemic collateral vessels (MAPCAs) to deliver blood to the lungs for oxygenation. These MAPCAs usually arise from the de- scending aorta and subclavian arteries. Commonly, the pulmonary arteries are abnormal, with hypoplastic (small and underdeveloped) central and branch pulmonary arteries and/ or non-confluent central pulmonary arteries. -

The Functional Anatomy of the Heart. Development of the Heart, Anomalies
The functional anatomy of the heart. Development of the heart, anomalies Anatomy and Clinical Anatomy Department Anastasia Bendelic Plan: Cardiovascular system – general information Heart – functional anatomy Development of the heart Abnormalities of the heart Examination in a living person Cardiovascular system Cardiovascular system (also known as vascular system, or circulatory system) consists of: 1. heart; 2. blood vessels (arteries, veins, capillaries); 3. lymphatic vessels. Blood vessels Arteries are blood vessels that carry blood away from the heart. Veins carry blood back towards the heart. Capillaries are tiny blood vessels, that connect arteries to veins. Lymphatic vessels: lymphatic capillaries; lymphatic vessels (superficial and deep lymph vessels); lymphatic trunks (jugular, subclavian, bronchomediastinal, lumbar, intestinal trunks); lymphatic ducts (thoracic duct and right lymphatic duct). Lymphatic vessels Microcirculation Microcirculatory bed comprises 7 components: 1. arterioles; 2. precapillaries or precapillary arterioles; 3. capillaries; 4. postcapillaries or postcapillary venules; 5. venules; 6. lymphatic capillaries; 7. interstitial component. Microcirculation The heart Heart is shaped as a pyramid with: an apex (directed downward, forward and to the left); a base (facing upward, backward and to the right). There are four surfaces of the heart: sternocostal (anterior) surface; diaphragmatic (inferior) surface; right pulmonary surface; left pulmonary surface. External surface of the heart The heart The heart has four chambers: right and left atria; right and left ventricles. Externally, the atria are demarcated from the ventricles by coronary sulcus (L. sulcus coronarius). The right and left ventricles are demarcated from each other by anterior and posterior interventricular sulci (L. sulci interventriculares anterior et posterior). Chambers of the heart The atria The atria are thin-walled chambers, that receive blood from the veins and pump it into the ventricles. -
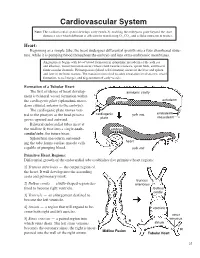
Cardiovascular System Note: the Cardiovascular System Develops Early (Week-3), Enabling the Embryo to Grow Beyond the Short
Cardiovascular System Note: The cardiovascular system develops early (week-3), enabling the embryo to grow beyond the short distances over which diffusion is efficient for transferring 2O , CO2, and cellular nutrients & wastes. Heart: Beginning as a simple tube, the heart undergoes differential growth into a four chambered struc- ture, while it is pumping blood throughout the embryo and into extra-embryonic membranes. Angiogenesis begins with blood island formation in splanchnic mesoderm of the yolk sac and allantois. Vessel formation occurs when island vesicles coalesce, sprout buds, and fuse to form vascular channels. Hematopoiesis (blood cell formation) occurs in the liver and spleen and later in the bone marrow. The transition from fetal to adult circulation involves new vessel formation, vessel merger, and degeneration of early vessels. Formation of a Tubular Heart: The first evidence of heart develop- amnionic cavity ment is bilateral vessel formation within ectoderm the cardiogenic plate (splanchnic meso- embryo derm situated anterior to the embryo). The cardiogenic plate moves ven- tral to the pharynx as the head process cardiogenic yolk sac endoderm mesoderm grows upward and outward. plate Bilateral endocardial tubes meet at the midline & fuse into a single endo- embryo cardial tube, the future heart. Splanchnic mesoderm surround- ing the tube forms cardiac muscle cells heart capable of pumping blood. yolk sac Primitive Heart Regions: Differential growth of the endocardial tube establishes five primitive heart regions: 1] Truncus arteriosus — the output region of the heart. It will develop into the ascending aorta and pulmonary trunk. truncus 2] Bulbus cordis — a bulb-shaped region des- arteriosus tined to become right ventricle. -

Functional Morphology of the Cardiac Jelly in the Tubular Heart of Vertebrate Embryos
Review Functional Morphology of the Cardiac Jelly in the Tubular Heart of Vertebrate Embryos Jörg Männer 1,*,† and Talat Mesud Yelbuz 2,† 1 Group Cardio‐Embryology, Institute of Anatomy and Embryology UMG, Georg‐August‐University Goettingen, D‐37075 Goettingen, Germany; [email protected] 2 Department of Cardiac Sciences, King Abdulaziz Cardiac Center, Section of Pediatric Cardiology, King Abdulaziz Medical City, Ministry of National Guard Health Affairs, Riyadh 11426, Saudi Arabia; [email protected] * Correspondence: [email protected]; Tel.: +49‐551‐39‐7032 † This work is dedicated to the memory of our academic mentors Gerd Steding (1936–2011) and Armin Wessel (1946–2011). Received: 29 January 2019; Accepted: 21 February 2019; Published: 27 February 2019 Abstract: The early embryonic heart is a multi‐layered tube consisting of (1) an outer myocardial tube; (2) an inner endocardial tube; and (3) an extracellular matrix layer interposed between the myocardium and endocardium, called “cardiac jelly” (CJ). During the past decades, research on CJ has mainly focused on its molecular and cellular biological aspects. This review focuses on the morphological and biomechanical aspects of CJ. Special attention is given to (1) the spatial distribution and fiber architecture of CJ; (2) the morphological dynamics of CJ during the cardiac cycle; and (3) the removal/remodeling of CJ during advanced heart looping stages, which leads to the formation of ventricular trabeculations and endocardial cushions. CJ acts as a hydraulic skeleton, displaying striking structural and functional similarities with the mesoglea of jellyfish. CJ not only represents a filler substance, facilitating end‐systolic occlusion of the embryonic heart lumen. -

Track 5: Cardiology and the Imaging Revolution
TRACK 5: CARDIOLOGY AND THE IMAGING REVOLUTION Volume 10 • Number 1 Abstract no: 1 Summer 2013 Real time 3-D echocardiographic characteristics of left ventricle and left atrium in normal children Bao Phung Tran Cong, Nii Masaki, Miyakoshi Chihiro, Yoshimoto Jun, Kato Atsuko, Ibuki Keichiro, Kim Sunghae, Mitsushita Norie, Tanaka Yasuhiko and Ono Yasuo Cardiac Department, Shizuoka Children’s Hospital, Shizuoka, Japan Background: The accurate assessment of left atrial (LA) and/or left ventricular (LV) volume and contractility is crucial for the management of patients with congenital heart disease. The real time 3-dimensional echocardiography (RT3-DE) is reported to show better correlation with magnetic resonance imaging (MRI) in estimating LV and LA volume than conventional 2-dimensional echocardiography (2-DE). On the other hand, the volume measurement in RT3-DE is also reported to be significantly smaller than those in MRI, necessitating the establishment of normal values of RT3-DE itself. Aim: To identify the normal values of LV and LA volume measured by RT3-DE in Japanese children. Methods: Sixty four normal school students (age: median 9.6 years; range (5.5 - 14.5); male 26, female 38) were enrolled in this study. End-diastolic and end- systolic LV and LA volumes were analysed using M-mode in short-axis view, 2-D biplane method, and RT3-DE. We used IE-33 (PHILIPS) with matrix probe X7 and X4. Off-line assessment to calculate LA and LV volume was done using QLAB 8.1 (Philips). Results: Forty nine children (age: median 9.1 years, range (6 - 14); male 21, female 28) had adequate RT3-DE data sets and were analysed.