Revision Guide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

B. Sample Multiple Choice Questions 1
Genetics Review B. Sample Multiple Choice Questions 1. A represents the dominant allele and a represents the recessive allele of a pair. If, in 1000 offspring, 500 are aa and 500 are of some other genotype, which of the following are most probably the genotypes of the parents? a. Aa and Aa b. Aa and aa c. AA and Aa d. AA and aa e. aa and aa 2. A form of vitamin D-resistant rickets, known as hypophosphatemia, is inherited as an X-linked dominant trait. If a male with hypophosphatemia marries a normal female, which of the following predictions concerning their potential progeny would be true? a. All of their sons would inherit the disease b. All of their daughters would inherit the disease c. About 50% of their sons would inherit the disease d. About 50% of their daughters would inherit the disease e. None of their daughters would inherit the disease 3. Which of the following best describes the parents in a testcross? a. One individual has the dominant phenotype and the other has the recessive phenotype. b. Both individuals are heterozygous. c. Both individuals have the dominant phenotype. d. Both individuals have the recessive phenotype. e. Both individuals have an unknown phenotype. 4. Which of the following is the most likely explanation for a high rate of crossing-over between two genes? a. The two genes are far apart on the same chromosome. b. The two genes are both located near the centromere. c. The two genes are sex-linked. d. The two genes code for the same protein. -

3Rd Period Allele: Alternate Forms of a Genetic Locus
Glossary of Terms Commonly Encountered in Plant Breeding 1st Period - 3rd Period Allele: alternate forms of a genetic locus. For example, at a locus determining eye colour, an individual might have the allele for blue eyes, brown, etc. Breeding: the intentional development of new forms or varieties of plants or animals by crossing, hybridization, and selection of offspring for desirable characteristics Chromosome: the structure in the eukaryotic nucleus and in the prokaryotic cell that carries most of the DNA Cross-over: The point along the meiotic chromosome where the exchange of genetic material takes place. This structure can often be identified through a microscope Crossing-over: The reciprocal exchange of material between homologous chromosomes during meiosis, which is responsible for genetic recombination. The process involves the natural breaking of chromosomes, the exchange of chromosome pieces, and the reuniting of DNA molecules Domestication: the process by which plants are genetically modified by selection over time by humans for traits that are more desirable or advantageous for humans DNA: an abbreviation for “deoxyribose nucleic acid”, the carrier molecule of inherited genetic information Dwarfness: The genetically controlled reduction in plant height. For many crops, dwarfness, as long as it is not too extreme, is an advantage, because it means that less of the crop's energy is used for growing the stem. Instead, this energy is used for seed/fruit/tuber production. The Green Revolution wheat and rice varieties were based on dwarfing genes Emasculation: The removal of anthers from a flower before the pollen is shed. To produce F1 hybrid seed in a species bearing monoecious flowers, emasculation is necessary to remove any possibility of self-pollination Epigenetic: heritable variation caused by differences in the chemistry of either the DNA (methylation) or the proteins associated with the DNA (histone acetylation), rather than in the DNA sequence itself Gamete: The haploid cell produced by meiosis. -

12.2 Characteristics and Traits
334 Chapter 12 | Mendel's Experiments and Heredity 12.2 | Characteristics and Traits By the end of this section, you will be able to do the following: • Explain the relationship between genotypes and phenotypes in dominant and recessive gene systems • Develop a Punnett square to calculate the expected proportions of genotypes and phenotypes in a monohybrid cross • Explain the purpose and methods of a test cross • Identify non-Mendelian inheritance patterns such as incomplete dominance, codominance, recessive lethals, multiple alleles, and sex linkage Physical characteristics are expressed through genes carried on chromosomes. The genetic makeup of peas consists of two similar, or homologous, copies of each chromosome, one from each parent. Each pair of homologous chromosomes has the same linear order of genes. In other words, peas are diploid organisms in that they have two copies of each chromosome. The same is true for many other plants and for virtually all animals. Diploid organisms produce haploid gametes, which contain one copy of each homologous chromosome that unite at fertilization to create a diploid zygote. For cases in which a single gene controls a single characteristic, a diploid organism has two genetic copies that may or may not encode the same version of that characteristic. Gene variants that arise by mutation and exist at the same relative locations on homologous chromosomes are called alleles. Mendel examined the inheritance of genes with just two allele forms, but it is common to encounter more than two alleles for any given gene in a natural population. Phenotypes and Genotypes Two alleles for a given gene in a diploid organism are expressed and interact to produce physical characteristics. -

Lecture No. IV
Course: Fundamentals of Genetics Class: - Ist Year, IInd Semester Lecture No. IV Title of topic: - Monohybrid crosses, Di-hybrid crosses, Test cross and Back cross Prepared by- Vinod Kumar, Assistant Professor, (PB & G) College of Agriculture, Powarkheda Monohybrid crosses:—Crosses between parents that differed in a single characteristic. The character (S) being studied in a monohybrid cross are governed by two or multiple variations for a single locus. The Mendel’s first law i.e. Law of segregation or purity of gametes can be explained by considering the monohybrid ratio i.e. by studying inheritance of only one character. For example: In pea, round seed shape is dominant over wrinkled seed shape. Generation Parental Parents Female X Male Phenotype Round X Wrinkled Genotype RR X rr Gametes R r Generation F1 Rr (Heterozygous) Round On selfing: Parents Female X Male Phenotype Round X round Genotype Rr X Rr Gametes R r R r Generation F2 ♂ R r ♀ RR Rr R Round Round r Rr rr Round Wrinkled Phenotype ratio: 3 round : 1 wrinkled Genotypic ratio: 1 RR : 2Rr : 1rr Two different alleles of the same gene i.e. ‘R’ and ‘r’ were brought together in the hybrid (F1). Even though the hybrid was round seeded in the next generation (F2) it produced both round and wrinkled seeded progeny. Thus both the alleles for round shape (R) and wrinkled shape (r) remained together in the hybrid without contaminating each other. In F2 generation (selfing of (F1) hybrid), the different phenotypes could be recovered because the two alleles in F1 remained pure and did not contaminate each other thus producing two types of gametes from F1 i.e. -
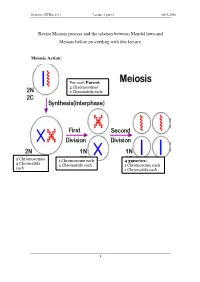
Revise Meiosis Process and the Relation Between Mendel Laws and Meiosis Before Proceeding with This Lecture
Genetics (BTBio 211) Lecture 3 part 2 2015-2016 Revise Meiosis process and the relation between Mendel laws and Meiosis before proceeding with this lecture. Meiosis Action: For each Parent 2 Chromosomes 2 Chromatids each 2 Chromosomes 1 Chromosome each 4 gametes: 4 Chromatid s 4 Chromatids each 1 Chromosome each each 1 Chromatids each 1 Genetics (BTBio 211) Lecture 3 part 2 2015-2016 LINKAGE AND CHROMOSOME MAPPING IN EUKARYOTES I. LINKAGE Genetic linkage is the tendency of genes that are located proximal to each other on a chromosome to be inherited together during meiosis. Genes whose loci are nearer to each other are less likely to be separated onto different chromatids during chromosomal crossover, and are therefore said to be genetically linked. Linked genes: Genes that are inherited together with other gene(s) in form of single unit as they are located on the same chromosome. For example: in fruit flies the genes for eye color and the genes for wing length are on the same chromosome, thus are inherited together. A couple of genes on chromosomes may be present either on the different or on the same chromosome. 1. The independent assortment of two genes located on different chromosomes. Mendel’s Law of Independent Assortment: during gamete formation, segregation of one gene pair is independent of other gene pairs because the traits he studied were determined by genes on different chromosomes. Consider two genes A and B, each with two alleles A a and B b on separate (different) chromosomes. 2 Genetics (BTBio 211) Lecture 3 part 2 2015-2016 Gametes of non-homologous chromosomes assort independently at anaphase producing 4 different genotypes AB, ab, Ab and aB with a genotypic ratio 1:1:1:1. -

7.014 Problem Set 6 Solutions
MIT Department of Biology 7.014 Introductory Biology, Spring 2004 7.014 Problem Set 6 Solutions Question 1 a) Define the following terms: Dominant – In genetics, the ability of one allelic form of a gene to determine the phenotype of a heterozygous individual, in which the homologous chromosomes carries both it and a different (recessive) allele. Recessive – In genetics, an allele that does not determine phenotype in the presence of a dominant allele. Phenotype – The observable properties of an individual resulting from both genetic and environmental factors. Genotype – An exact description of the genetic constitution of an individual, either with respect to a single trait or with respect to a larger set of traits. Alleles – The alternate forms of a genetic character found at a given locus on a chromosome. Homozygous – In a diploid organism, having identical alleles of a given gene on both homologous chromosomes. An individual may be a homozygote with respect to one gene and a heterozygote with respect to another. Heterozygous – Of a diploid organism having different alleles of a given gene on the pair of homologues carrying that gene. Mendel’s First Law – Law of Segregation - In genetics, the separation of alleles, or of homologous chromosomes, from one another during meiosis so that each of the haploid daughter nuclei produced by meiosis contains one or the other member of the pair found in the diploid mother cell, but never both. Mendel’s Second Law – Law of Independent Assortment - During meiosis, the random separation of genes carried on nonhomologous chromosomes. Sex-Linked – The pattern of inheritance characteristic of genes located on the sex chromosomes of organisms having a chromosomal mechanism for sex determination. -

Dihybrid Crosses
Dihybrid Crosses: Punnett squares for two traits Genes on Different Chromosomes If two genes are on different chromosomes, all four possible alleles combinations for two different genes in a heterozygous cross are _____________ due to independent assortment. If parents are RrYy (heterozygous for both traits) Equally R r likely to R r OR line up either way y Y Y y when dividing Ry rY RY ry Gametes: ___________________________________ Setting up a Dihybrid Cross: RrYy x RrYy Each side of a Punnett Square represents all the possible allele combinations in a gamete from a parent. Parent gametes always contain one allele for___ _______ .(____________-R or r & Y or y in this case). Four possible combinations of the alleles for the two genes are possible if heterozygous for both traits. (For example: ___________________) Due to independent assortment, each possible combination is equally likely if genes are on separate chromosomes. Therefore Punnett squares indicate probabilities for each outcome. Discuss with your table partner: A. R r Y y Which is correct for a R dihybrid cross of two r heterozygous parents RrYy x RrYy? Y y B. RR Rr rr rR C. RY Ry rY ry YY RY Yy Ry yy rY yY ry Explain how the correct answer relates to the genes passed down in each gamete (egg or sperm) Dihybrid Cross of 2 Heterozygotes 9 3 3 1 Heterozygous Dihybrid Cross Dominant Dominant Recessive Recessive for both 1st trait 1st trait for both traits Recessive Dominant traits 2nd trait 2nd trait Round Round Wrinkled Wrinkled Yellow Green Yellow Green __ __ __ __ ______ = ______ = ______ = _______ = ___ ____ ____ _____ Heterozygous cross __________ratio if independent assortment Mendel came up with the Law of Independent Assortment because he realized that the results for his dihybrid crosses matched the probability of the two genes being inherited independently. -

Genetics Slides
Genetics - part one Simple Genetics (One Gene for One Trait) n Mendel’s Pea Plants. – Tall or short. – Round or wrinkled. – Etc… n Dominant & recessive. n Purebred or Hybrid. n Simple Punnett Squares. Gregor Mendel TEST 1 on SLIDES 1-19 1 n Gregor Mendel was a monk who worked with pea plants in the mid 1800’s. Today he is known as “Father of Genetics.” He was curious about the “factors” acting on inheritance. Are there patterns? 2 What is Genetics? n Genetics is the branch of biology that deals with heredity. n Heredity is the passing of traits from parents to offspring. – Parents à offspring n Traits are characteristics of an organism. – Human examples: height, hair, eye color. 3 1 Genetics - part one n Gene: a section/piece of DNA on a chromosome that codes for a specific trait. § Alleles = alternate forms of a gene (Ex: Blood type A/B/O). § Genes tend to come in pairs (alleles). n DOMINANT: an allele whose trait always shows up in the organism when the allele present. – (CAPITAL LETTER) n recessive: an allele hidden by dominant. – (lower case letter) n Genotype: genetic makeup; allele combinations. – (Ex: BB, Bb, bb) n Phenotype: physical appearance of an organism (Ex: Brown hair) 4 How to make a test cross (aka Punnett Square) Parent #1 alleles Offspring Offspring Offspring Offspring Parent #2 alleles 5 6 2 Genetics - part one Diagrams & math can be used to predict the probability that a trait will pass from parent(s) to offspring. Punnet Squares 7 8 Complete this test cross between two purebred pea plants: T T t t T = tall stem t = short stem 9 3 Genetics - part one 10 11 Where are genes? n Genes & chromosomes come in pairs. -

In This Lesson, You Will Learn How to Predict the Probable Genetic Makeup and Appearance of Offspring Resulting from Specific Cr
In this lesson, you will learn how to predict the probable genetic makeup and appearance of offspring resulting from specific crosses.! Probability! n Predicts the likelihood that a specific event will occur.! n May be expressed a a decimal, a percentage, or a fraction.! n Determined by the following formula:! Probability = number of times an event is expected to happen! number of opportunities for an event to happen! Why probability is important to genetics! n Mendel used probability to determine how likely the dominant trait would appear over the recessive trait.! n The yellow pea appeared 6,022 times in the F2 generation. The green pea appeared 2,001 times. ! n The total number of individual was 8023 (6022+2001)! n Using the formula: ! n 6022 ÷ 8023 = 0.75 ! n 2001 ÷ 8023 = 0.25 ! n Percentage: 75% green peas 25% yellow peas! n Ratio: 3:1 ratio of yellow to green peas! n Fraction: 1/4 chance of green peas and 3/4 chance of yellow peas! ! ! Results of the F1 generation! PP Pp x 2 pp ratio: 1:2:1 or 3:1 purple to white! 75% purple to 25% white! F1 generation yielded 100% purple flowers, heterozygous for the purple trait.! white (pp) x purple(PP) ! ! ! ! yields! ! ! ! 100% purple flowers that are heterozygous for the purple flower trait (Pp).! Punnett Square! n A diagram used to predict the probability of certain traits by offspring.! n The following examples will illustrate the outcome of different types of crosses.! How to set up and work a Punnett square! n Draw a four-square box.! n Place one set of alleles on the side of the box as shown at right.! How to set up and work a Punnett square! n One set of alleles for a trait go on top of the box (usually male) and the other set of alleles go on the side of the box.! n Each letter from the set of alleles is placed on top of the square.! Filling in the boxes…! n Fill in the top left box with the alleles from top left and upper left.! n The dominant letter is placed first. -
Genetics Worksheet
Genetics Worksheet Part 1 Introduction: 1. Describe the genotypes given (use your notes). The first two are already done. A. DD homozygous, dominant D. ss ______________________ B. Dd _heterozygous E. Yy ______________________ C. dd __________________ F. WW ____________________ 2. In humans, brown eye color (B), is dominant over blue eye color (b). What are the phenotypes of the following genotypes? In other words, what color eyes will they have? A. BB ________________________ B. bb ________________________ C. Bb ________________________ The Five (5) Steps Associated With Solving a Genetics Problem: If you take the time to follow the directions below, you will be able to solve most genetics problems. 1. Determine the genotypes of the parents or whatever is given in problem. 2. Set up your Punnett square as follows: *# sq. based on possible gametes that can be formed. Possiblepossible parental gametes gametes otherOther possible possible parentalparental gametes gametes 3. Fill in the squares. This represents the possible combinations that could occur during fertilization. 4. Write out the possible genotypic ratio of the offspring. 5. Using the genotypic ratio determine the phenotypic ratio for the offspring. Part 2: Sample Problem (Just read this over, it is a practice problem) A heterozygous male, black eyed mouse is crossed with a red eyed, female mouse. Predict the possible offspring! Step 1: Determine the genotype of the parents. The male parent is heterozygous which means he has one allele for black eyes and one allele for red eyes. Since his eyes are black, this means that black allele must be dominant over the red allele. -
Ch 14 Review Questions
Genetics I Answered Review Questions 1. Who discovered the laws of heredity and with what specific organism was this person working? “Modern genetics had its genesis in an abbey garden, where a monk named Gregor Mendel documented a particulate mechanism of inheritance. The painting in the figure below, depicts Mendel working with his experimental organism, garden peas. Mendel developed his theory of inheritance several decades before the behavior of chromosomes was observed in the microscope and their significance understood.” (Text quoted from page 251 of the textbook) 2. What are alleles and where would they be located in a cell? “…alternative versions of genes account for variations in inherited characters. The gene for flower color in pea plants, for example, exists in two versions, one for purple flowers and the other for white. These alternative versions of a gene are now called alleles (see table below). Today, we can relate this concept to chromosomes and DNA. As noted in Chapter 13, each gene resides at a specific locus on a specific chromosome. The DNA at that locus, however, can vary somewhat in its sequence of nucleotides and hence in its information content. The purple-flower allele and the white-flower allele are two DNA variations possible at the flower-color locus on one of a pea plant’s chromosomes.” (Text quoted from pages 253 and 254 of the textbook.) 3. Contrast dominant and recessive alleles. “if the two alleles at a locus differ, then one, the dominant allele, determines the organism’s appearance; the other, the recessive allele, has no noticeable effect on the organism’s appearance.” (Text quoted from page 254 of the textbook) A dominant allele may be expressed when only one dominant allele is present. -
Mendelian Gene[Cs & the Chromosome Theory of Inheritance
Mendelian Genecs & the Chromosome Theory of Inheritance • Mendel 1840s-1860s – Ignored Completely • Darwin 1860 – Theory incompable with blending model of inheritance. • Microscopy techniques constantly improving • Mitosis discovered in 1870s • Meiosis discovered in 1890s • In 1900s Mendel’s work rediscovered! • Walter Suon & Theodor Boveri recognized the parallels between Mendel’s work and how chromosomes behaved during meiosis. Meiosis & Chromosomes 1. Chromosomes present in 1. Genes present in pairs in diploid cells. pairs in diploid cells. 2. Homologous chromosomes 2. Alleles segregate separate during meiosis. during meiosis. 3. Ferlizaon restores paired 3. Ferlizaon restores condion for chromosomes. paired condion for genes. Figure 15.2a P Generation Yellow-round Green-wrinkled seeds (YYRR) seeds (yyrr) Y y × r R R r Y y Meiosis Fertilization R Y y r Gametes Figure 15.2b All F1 plants produce yellow-round seeds (YyRr). F1 Generation R R y y r r Y Y LAW OF INDEPENDENT Meiosis LAW OF SEGREGATION ASSORTMENT Alleles of The two alleles for each R r r R genes on nonhomologous gene separate during chromosomes assort gamete formation. Metaphase I independently during gamete Y y Y y formation. 1 1 R r r R Anaphase I Y y Y y R r Metaphase r R 2 II 2 Y y Y y y Y Y Y y Gametes Y y y R r R r r r R R 1 1 1 1/ /4 YR /4 yr /4 Yr 4 yR Figure 15.2c LAW OF SEGREGATION LAW OF INDEPENDENT ASSORTMENT F2 Generation An F1 × F1 cross-fertilization 3 Fertilization 3 Fertilization results recombines the in the 9:3:3:1 R and r alleles 9 : 3 : 3 : 1 phenotypic ratio in at random.