Bioactive Components and Pharmacological Effects of Canna Indica- an Overview
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Somatic Embryogenesis and Genetic Fidelity Study of Micropropagated Medicinal Species, Canna Indica
Horticulturae 2015, 1, 3-13; doi:10.3390/horticulturae1010003 OPEN ACCESS horticulturae ISSN 2311-7524 www.mdpi.com/journal/horticulturae Article Somatic Embryogenesis and Genetic Fidelity Study of Micropropagated Medicinal Species, Canna indica Tanmayee Mishra 1, Arvind Kumar Goyal 2 and Arnab Sen 1,* 1 Molecular Cytogenetics Laboratory, Department of Botany, University of North Bengal, Siliguri 734013, West Bengal, India; E-Mail: [email protected] 2 Bamboo Technology, Department of Biotechnology, Bodoland University, Kokrajhar 783370, Assam, India; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +91-353-269-9118; Fax: +91-353-269-9001. Academic Editors: Douglas D. Archbold and Kazumi Nakabayashi Received: 23 February 2015 / Accepted: 30 April 2015 / Published: 8 May 2015 Abstract: Canna indica Linn. (Cannaceae), is used both as medicine and food. Traditionally, various parts of C. indica are exploited to treat blood pressure, dropsy, fever, inflammatory diseases etc. However, till date there is no reliable micropropagation protocol for C. indica. We present here a regeneration technique of C. indica with banana micropropagation medium (BM). BM supplemented with 3% sucrose, 0.7% agar, −1 and 0.17% NH4NO3 and different plant growth regulators like BAP (2 mg·L ) and NAA (0.5 mg·L−1) was found to be effective in inducing callus in C. indica. BM with BAP (2 mg·L−1) was ideal for somatic embryogenesis and plantlet regeneration. After a period of 3 months, regenerated plantlets were successfully transferred to the field conditions. Appearance of somaclonal variation among the regenerated plants is a common problem which could be assessed by DNA fingerprinting. -

Resolution of Deep Angiosperm Phylogeny Using Conserved Nuclear Genes and Estimates of Early Divergence Times
ARTICLE Received 24 Mar 2014 | Accepted 11 Aug 2014 | Published 24 Sep 2014 DOI: 10.1038/ncomms5956 OPEN Resolution of deep angiosperm phylogeny using conserved nuclear genes and estimates of early divergence times Liping Zeng1, Qiang Zhang2, Renran Sun1, Hongzhi Kong3, Ning Zhang1,4 & Hong Ma1,5 Angiosperms are the most successful plants and support human livelihood and ecosystems. Angiosperm phylogeny is the foundation of studies of gene function and phenotypic evolution, divergence time estimation and biogeography. The relationship of the five divergent groups of the Mesangiospermae (B99.95% of extant angiosperms) remains uncertain, with multiple hypotheses reported in the literature. Here transcriptome data sets are obtained from 26 species lacking sequenced genomes, representing each of the five groups: eudicots, monocots, magnoliids, Chloranthaceae and Ceratophyllaceae. Phylogenetic analyses using 59 carefully selected low-copy nuclear genes resulted in highly supported relationships: sisterhood of eudicots and a clade containing Chloranthaceae and Ceratophyllaceae, with magnoliids being the next sister group, followed by monocots. Our topology allows a re-examination of the evolutionary patterns of 110 morphological characters. The molecular clock estimates of Mesangiospermae diversification during the late to middle Jurassic correspond well to the origins of some insects, which may have been a factor facilitating early angiosperm radiation. 1 State Key Laboratory of Genetic Engineering and Collaborative Innovation Center for Genetics and Development, Ministry of Education Key Laboratoryof Biodiversity Sciences and Ecological Engineering, Institute of Plant Biology, Institute of Biodiversity Science, Center for Evolutionary Biology, School of Life Sciences, Fudan University, 220 Handan Road, Yangpu District, Shanghai 200433, China. 2 Guangxi Institute of Botany, Guangxi Zhuang Autonomous Region and the Chinese Academy of Sciences, Guilin 541006, China. -
Current Catalog
PLATT HILL NURSERY 222 W. Lake St. 2400 Randall Road Bloomingdale, IL Carpentersville, IL 60108-1038 60110-3424 630-529-9394 847-428-6767 FAX: FAX: 630-529-3795 847-428-3812 www.platthillnursery.com 2020 CATALOG STORE HOURS: SPRING REGULAR (Mid-April – Mid-June) Mon-Fri 9-8 Mon-Sat 9-6 Sat 9-6 Sun 10-5 Sun 9-6 MISSION STATEMENT Platt Hill Nursery is a complete retail garden center where meeting our customers’ needs is our first priority. Our mission is to: • Consistently provide our customers with professional service combined with the best quality and widest selection of plants, garden and seasonal merchandise. • Create and maintain a positive working environment for the welfare and growth of the employees and the company. • Earn a fair profit as a return on investment. • Strive for excellence in everything we do and be the best garden center of our time. ❧ ❧ ❧ SEASONAL SPECIALTIES VALENTINE’S DAY - Blooming Plants SPRING - Bedding Plants, Vegetable Plants, Perennials, Roses, Trees, Shrubs, Seeds, Fertilizer, Tools, Trellises, Stone, Mulch, Tropical Blooming Plants EASTER - Lilies, Potted Tulips, Hyacinth, Blooming Plants, Flowering Easter Baskets MOTHER’S DAY - Blooming Plants, Flowering Garden Baskets, Annual Planters FATHER’S DAY - Patio Planters, Gardening Supplies, Bird Feeders, Fountains, Trees AUTUMN - Fall Mums, Pumpkins, Gourds, Indian Corn, Bulbs for Spring Color, Trees, Shrubs, Evergreens, Mulch CHRISTMAS - Poinsettias, Fresh Cut Christmas Trees, Fresh Wreaths and Roping, Artificial Trees, Artificial Wreaths and Roping, Light Sets, Bows, Decorations and More! WINTER - Birdseed, Firewood, Tropical Plants, Wicker Baskets, Decorative Pots, Spring Atmosphere ❧ ❧ ❧ GUARANTEE Platt Hill Nursery guarantees: • Trees and shrubs for 50% of the purchase price for one year from the date of purchase. -

Selected Sri Lankan Food Plants and Other Herbs As Potential Sources of Inulin-Type Fructans
J.Natn.Sci.Foundation Sri Lanka 2015 43 (1): 35 - 43 DOI: http://dx.doi.org/10.4038/jnsfsr.v43i1.7913 RESEARCH ARTICLE Selected Sri Lankan food plants and other herbs as potential sources of inulin-type fructans D.C. Mudannayake 1, K.M.S. Wimalasiri 2* , K.F.S.T. Silva 3 and S. Ajlouni 4 1 Department of Animal Science, Faculty of Animal Science and Export Agriculture, Uva Wellassa University, Badulla. 2 Department of Food Science and Technology, Faculty of Agriculture, University of Peradeniya, Peradeniya. 3 Department of Animal Science, Faculty of Agriculture, University of Peradeniya, Peradeniya. 4 Bioscience Section, Faculty of Veterinary and Agricultural Sciences, The University of Melbourne, Victoria 3010, Australia. Revised: 10 July 2014; Accepted: 29 August 2014 Abstract: The objective of this study was to determine the inulin-type fructan content in 20 selected food plants and fructose linkages with an optional terminating glucose other herbs commonly found in Sri Lanka. The inulin content molecule. They are considered linear or branched of the selected plants were determined qualitatively and fructose polymers with a degree of polymerization of quantitatively using thin layer chromatography (TLC) and 2-60 (Roberfroid, 2005; 2007b). enzymatic spectrophotometric (ES) methods, respectively. The ES results showed that the inulin-type fructan contents based on fresh weight was highest in Allium sativum (18.62 % Inulin-type fructans have gained much interest ± 1.55), followed by Asparagus falcatus (17.74 % ± 2.92), in the food industry as ‘functional food ingredients’ Asparagus racemosus (11.8 3% ± 0.87), Allium cepa (8.60 % because they have the ability to selectively stimulate ± 0.88), Allium ampeloprasum (6.20 % ± 0.23), Taraxacum the activities and growth of beneficial microflora javanicum (5.77 % ± 1.53) and Vernonia cinerea (4.55 % (specifically Bifidobacteria and some Lactobacillus ± 0.93), respectively. -

RAICES ANDINAS: Manual De Capacitación
Conservación y uso de la biodiversidad de raíces y tubérculos andinos: Una década de investigación para el desarrollo (1993-2003) 6 Raíces Andinas: Contribuciones al conocimiento y a la capacitación Editor Técnico: Juan Seminario RAICES ANDINAS: Contribuciones al conocimiento y a la capacitación 2004 Copyright: Los autores autorizan la reproducción total o parcial de esta publicación, dando el crédito correspondiente a los autores/instituciones e incluyendo la citación correcta de esta publicación. ISNB: 92-9060-233-3 Lima, Perú Lista de autores por instituciones, en orden alfabético: Centro de Servicios Centro Internacional Corporación Empresa Múltiples de Apoyo al de la Papa: Colombiana de Brasileira de Desarrollo de Semilla: Raúl Blas Investigación Pesquisa Julio Rea Patricio Espinosa Agropecuaria: Agropecuária: Roberto González Guillermo Caicedo Fausto Dos Michael Hermann Mario Lobo Santos Miguel Holle Clara Medina Charlotte Lizarraga Guillermo Sánchez Sonia Salas Luis Torres Andrés Valladolid Norma Vásquez The Royal Veterinary Universidad Mayor de Universidad Universidad Agricultural San Simón: Nacional de Nacional San University: Julio Espinoza Cajamarca: Cristóbal de Steen R. Knudsen Tony Coronel Huamanga: Martín Sorensen Isidoro Sánchez Fernando Juan Seminario Barrantes Miguel Valderrama Universidad Nacional Universidad Ricardo Mayor de San Marcos: Palma: Guido Ayala Carola Escobar David Talledo Editores Técnicos: Juan Seminario, Universidad Nacional de Cajamarca Editores de la Serie: Michael Hermann, Centro Internacional de la Papa Oscar A. Hidalgo, Agro Consult International S.A.C. Edición: Teresa Ames de Icochea, Centro Internacional de la Papa Coordinación: Cecilia Lafosse, Departamento de Comunicación y Difusión, CIP Carátula: Anselmo Morales, Departamento de Comunicación y Difusión, CIP Diagramación: Mercedes Suito, Departamento de Capacitación, CIP Centro Internacional de la Papa (CIP) Apartado 1558, La Molina Lima 12, Perú. -

The Evolutionary and Biogeographic Origin and Diversification of the Tropical Monocot Order Zingiberales
Aliso: A Journal of Systematic and Evolutionary Botany Volume 22 | Issue 1 Article 49 2006 The volutE ionary and Biogeographic Origin and Diversification of the Tropical Monocot Order Zingiberales W. John Kress Smithsonian Institution Chelsea D. Specht Smithsonian Institution; University of California, Berkeley Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Botany Commons Recommended Citation Kress, W. John and Specht, Chelsea D. (2006) "The vE olutionary and Biogeographic Origin and Diversification of the Tropical Monocot Order Zingiberales," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 22: Iss. 1, Article 49. Available at: http://scholarship.claremont.edu/aliso/vol22/iss1/49 Zingiberales MONOCOTS Comparative Biology and Evolution Excluding Poales Aliso 22, pp. 621-632 © 2006, Rancho Santa Ana Botanic Garden THE EVOLUTIONARY AND BIOGEOGRAPHIC ORIGIN AND DIVERSIFICATION OF THE TROPICAL MONOCOT ORDER ZINGIBERALES W. JOHN KRESS 1 AND CHELSEA D. SPECHT2 Department of Botany, MRC-166, United States National Herbarium, National Museum of Natural History, Smithsonian Institution, PO Box 37012, Washington, D.C. 20013-7012, USA 1Corresponding author ([email protected]) ABSTRACT Zingiberales are a primarily tropical lineage of monocots. The current pantropical distribution of the order suggests an historical Gondwanan distribution, however the evolutionary history of the group has never been analyzed in a temporal context to test if the order is old enough to attribute its current distribution to vicariance mediated by the break-up of the supercontinent. Based on a phylogeny derived from morphological and molecular characters, we develop a hypothesis for the spatial and temporal evolution of Zingiberales using Dispersal-Vicariance Analysis (DIVA) combined with a local molecular clock technique that enables the simultaneous analysis of multiple gene loci with multiple calibration points. -

Soil Service Garden Center, Inc. Canna Growing Guide
Canna Growing Guide Cannas are native to the northern and southern hemisphere. Cannas do well in most areas of the United States, but flourish with plenty of heat and water. Cannas are very dependable; easy to plant and easy to grow. Cannas offer showy, tropical color from early sum- mer until frost. Cannas are regaining much of the popularity they once enjoyed as an old garden favorite. PLANTING: Cannas may be planted in the spring after danger from hard frost. In zone 7 we recommend planting from late March to late April. Adjust this guideline to your zone. Before spring planting, soil can be amended with com- post, manure and a high nitrogen fertilizer. Best results are achieved when planted in a loose, fertile and well drained soil that has warmed to 60 degrees. Cannas will tolerate a wide range of growing conditions. Cannas love full sun and require a minimum of four hours of direct sunlight. Plant rhizomes 12 to 18 inches apart. Lay the long part of the rhizome horizontal to the earth’s surface with eye up, if visible. This is not critical, as cannas will grow no matter which direction they are planted. Cover with 2 inches of soil. In colder regions, (6-8 weeks before spring), bulbs can be planted in pots and placed in greenhouse conditions. When danger of frost is past, remove from pot and plant outside. Cultivate often to keep soil loose and free of weeds. WATERING & FERTILIZATION: Cannas should be watered thoroughly once a week by slowly soaking the area around roots. -

Crop Group Tables. Vegetables Group
Environmental Protection Agency § 180.41 appropriate times, EPA will amend tol- dividual tolerances must be estab- erances for crop groups that have been lished. Miscellaneous commodities in- superseded by revised crop groups to tentionally not included in any group conform the pre-existing crop group to include asparagus, avocado, banana, the revised crop group. Once all of the fig, globe artichoke, hops, mango, pa- tolerances for the pre-existing crop paya, pawpaw, peanut, persimmon, group have been updated, the pre-exist- pineapple, water chestnut, and water- ing crop group will be removed from cress. the CFR. (c) Each group is identified by a (k) Establishment of a tolerance does group name and consists of a list of not substitute for the additional need representative commodities followed to register the pesticide under a com- panion law, the Federal Insecticide, by a list of all commodity members for Fungicide, and Rodenticide Act. The the group. If the group includes sub- Registration Division of the Office of groups, each subgroup lists the sub- Pesticide Programs should be con- group name, the representative com- tacted concerning procedures for reg- modity or commodities, and the mem- istration of new uses of a pesticide. ber commodities for the subgroup. Sub- groups, which are a subset of their as- [60 FR 26635, May 17, 1995, as amended at 70 sociated crop group, are established for FR 33363, June 8, 2005; 72 FR 69155, Dec. 7, 2007; 75 FR 56014, Sept. 15, 2010] some but not all crops groups. (1) Crop Group 1: Root and Tuber § 180.41 Crop group tables. -

Pollutant Removal by Canna Generalis in Tropical Constructed Wetlands for Domestic Wastewater Treatment
Global J. Environ. Sci. Manage. 5(3): 331-344, Summer 2019 Global Journal of Environmental Science and Management (GJESM) Homepage: https://www.gjesm.net/ ORIGINAL RESEARCH PAPER Pollutant removal by Canna Generalis in tropical constructed wetlands for domestic wastewater treatment H.D. Tran1,*, H.M.T. Vi2, H.T.T. Dang1, R.M. Narbaitz3 1 Department of Water Supply and Sanitation, Faculty of Environmental Engineering, National University of Civil Engineering, Vietnam 2 Department of Environmental Engineering, Thai Nguyen University, Tan Thinh Ward, Thai Nguyen, Vietnam 3 Department of Civil Engineering, University of Ottawa, 161 Louis Paster Pvt., Ottawa k1N 6N5, Canada ARTICLE INFO ABSTRACT Article History: Constructed wetlands have not been commonly used in Vietnam due to the lack Received 24 January 2019 of information in the selection of proper types of constructed wetlands, type of Revised 23 April 2019 reeds, design parameters and performance efficiency, in tropical climates. This Accepted 24 May 2019 paper focuses on Canna generalis, which is a common reed and easy to grow both in water and wet land conditions. Two kinds of hybrid constructed wetlands were employed, including Facultative pond combined with free water sub-surface Keywords: constructed wetlands system and horizontal subsurface flow combined with Canna Generalis Aerobic pond system. It was found that the ponds played an important role in Constructed wetlands (CW) the hybrid system performance and enhanced the performance of constructed Free water subsurface (FWS) wetlands. -

Treatment of Various Diseases by Canna Indica L
Online - 2455-3891 Vol 11, Issue 12, 2018 Print - 0974-2441 Review Article TREATMENT OF VARIOUS DISEASES BY CANNA INDICA L. - A PROMISING HERB VANITA KANASE*, SUNITA VISHWAKARMA Department of Pharmacology, Oriental College of Pharmacy, Sanpada, Navi Mumbai, Maharashtra, India. Email: [email protected] Received: 02 July 2018, Revised and Accepted: 21 August 2018 ABSTRACT In recent years, ethnobotanical and traditional application of natural compounds, principally of plant origin established much attention as they are well tested for their effectiveness and generally believed to be non-toxic for human use. Canna indica L. is a tropical herb belonging to the family Cannaceae. It has been extensively used in a traditional remedy for the treatment of many complaints. The phytochemical analysis of C. indica exhibited that it contained various phytochemicals including alkaloids, cardiac glycosides, anthocyanin pigments, flavonoids, steroids, terpenoids, tannins, phlobatannins, saponins, carbohydrates, proteins, oils, and many other chemical compounds. The pharmacological studies showed that this plant exerted anthelmintic, antibacterial, antimicrobial, antiviral, antidiabetic, antidiarrheal, anti-inflammatory, analgesic, immunomodulatory, antioxidant, cytotoxic, hemostatic, hepatoprotective, molluscicidal, and other effects. This review attempts to illuminate the available literature on C. indica (L.) with respect to ethnobotany, chemical constituents, and summary of numerous pharmacological activities. Every part of C. indica has valuable -
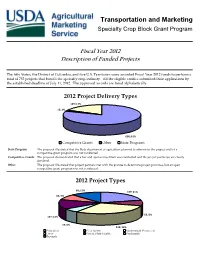
SCBGP-FB (08FB- 12) Description of Funded Projects
Transportation and Marketing Specialty Crop Block Grant Program Fiscal Year 2012 Description of Funded Projects The fifty States, the District of Columbia, and five U.S. Territories were awarded Fiscal Year 2012 funds to perform a total of 752 projects that benefit the specialty crop industry. All the eligible entities submitted their applications by the established deadline of July 11, 2012. The approved awards are listed alphabetically. 2012 Project Delivery Types 129; 17% 15; 2% 608; 81% Competitive Grants Other State Programs State Program The proposal illustrated that the State department of agriculture planned to administer the project and/or a competitive grant program was not conducted. Competitive Grants The proposal demonstrated that a fair and open competition was conducted and the project partner(s) are clearly involved. Other The proposal illustrated that project partners met with the grantee to determine project priorities, but an open competitive grant program was not conducted. 2012 Project Types 88; 12% 157; 21% 64; 9% 63; 8% 107; 14% 43; 6% 230; 30% Education Food Safety Marketing & Promotion Other Pest & Plant Health Production Research Alabama Department of Agriculture and Industries Amount Awarded: $401,366.76 Number of Projects: 15 • Partner with Tuskegee University to increase the number of specialty crop producers who have completed farm food safety plans by providing educational opportunities and providing a cost-share for food safety certification for some of the participants • Partner with the Cullman County -

Networks in a Large-Scale Phylogenetic Analysis: Reconstructing Evolutionary History of Asparagales (Lilianae) Based on Four Plastid Genes
Networks in a Large-Scale Phylogenetic Analysis: Reconstructing Evolutionary History of Asparagales (Lilianae) Based on Four Plastid Genes Shichao Chen1., Dong-Kap Kim2., Mark W. Chase3, Joo-Hwan Kim4* 1 College of Life Science and Technology, Tongji University, Shanghai, China, 2 Division of Forest Resource Conservation, Korea National Arboretum, Pocheon, Gyeonggi- do, Korea, 3 Jodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, United Kingdom, 4 Department of Life Science, Gachon University, Seongnam, Gyeonggi-do, Korea Abstract Phylogenetic analysis aims to produce a bifurcating tree, which disregards conflicting signals and displays only those that are present in a large proportion of the data. However, any character (or tree) conflict in a dataset allows the exploration of support for various evolutionary hypotheses. Although data-display network approaches exist, biologists cannot easily and routinely use them to compute rooted phylogenetic networks on real datasets containing hundreds of taxa. Here, we constructed an original neighbour-net for a large dataset of Asparagales to highlight the aspects of the resulting network that will be important for interpreting phylogeny. The analyses were largely conducted with new data collected for the same loci as in previous studies, but from different species accessions and greater sampling in many cases than in published analyses. The network tree summarised the majority data pattern in the characters of plastid sequences before tree building, which largely confirmed the currently recognised phylogenetic relationships. Most conflicting signals are at the base of each group along the Asparagales backbone, which helps us to establish the expectancy and advance our understanding of some difficult taxa relationships and their phylogeny.