Epithelial Control of Gut-Associated Lymphoid Tissue Formation Through P38α-Dependent Restraint of NF-Κb Signaling
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Herbivore Digestive System Buffalo Zebra
The Herbivore Digestive System Name__________________________ Buffalo Ruminant: The purpose of the digestion system is to ______________________________ _____________________________. Bacteria help because they can digest __________________, a sugar found in the cell walls of________________. Zebra Non- Ruminant: What is the name for the largest section of Organ Color Key a ruminant’s Mouth stomach? Esophagus __________ Stomach Small Intestine Cecum Large Intestine Background Information for the Teacher Two Strategies of Digestion in Hoofed Mammals Ruminant Non‐ruminant Representative species Buffalo, cows, sheep, goats, antelope, camels, Zebra, pigs, horses, asses, hippopotamus, rhinoceros giraffes, deer Does the animal Yes, regurgitation No regurgitation regurgitate its cud to Grass is better prepared for digestion, as grinding Bacteria can not completely digest cell walls as chew material again? motion forms small particles fit for bacteria. material passes quickly through, so stool is fibrous. Where in the system do At the beginning, in the rumen Near the end, in the cecum you find the bacteria This first chamber of its four‐part stomach is In this sac between the two intestines, bacteria digest that digest cellulose? large, and serves to store food between plant material, the products of which pass to the rumination and as site of digestion by bacteria. bloodstream. How would you Higher Nutrition Lower Nutrition compare the nutrition Reaps benefits of immediately absorbing the The digestive products made by the bacteria are obtained via digestion? products of bacterial digestion, such as sugars produced nearer the end of the line, after the small and vitamins, via the small intestine. intestine, the classic organ of nutrient absorption. -

Mouth Esophagus Stomach Rectum and Anus Large Intestine Small
1 Liver The liver produces bile, which aids in digestion of fats through a dissolving process known as emulsification. In this process, bile secreted into the small intestine 4 combines with large drops of liquid fat to form Healthy tiny molecular-sized spheres. Within these spheres (micelles), pancreatic enzymes can break down fat (triglycerides) into free fatty acids. Pancreas Digestion The pancreas not only regulates blood glucose 2 levels through production of insulin, but it also manufactures enzymes necessary to break complex The digestive system consists of a long tube (alimen- 5 carbohydrates down into simple sugars (sucrases), tary canal) that varies in shape and purpose as it winds proteins into individual amino acids (proteases), and its way through the body from the mouth to the anus fats into free fatty acids (lipase). These enzymes are (see diagram). The size and shape of the digestive tract secreted into the small intestine. varies in each individual (e.g., age, size, gender, and disease state). The upper part of the GI tract includes the mouth, throat (pharynx), esophagus, and stomach. The lower Gallbladder part includes the small intestine, large intestine, The gallbladder stores bile produced in the liver appendix, and rectum. While not part of the alimentary 6 and releases it into the duodenum in varying canal, the liver, pancreas, and gallbladder are all organs concentrations. that are vital to healthy digestion. 3 Small Intestine Mouth Within the small intestine, millions of tiny finger-like When food enters the mouth, chewing breaks it 4 protrusions called villi, which are covered in hair-like down and mixes it with saliva, thus beginning the first 5 protrusions called microvilli, aid in absorption of of many steps in the digestive process. -

6-Physiology of Large Intestine.Pdf
LARGE INTESTINE COLON MOTILITY Color index • Important • Further explanation 1 Contents . Mind map.......................................................3 . Colon Function…………………………………4 . Physiology of Colon Regions……...…………6 . Absorption and Secretion…………………….8 . Types of motility………………………………..9 . Innervation and motility…………………….....11 . Defecation Reflex……………………………..13 . Fecal Incontinence……………………………15 Please check out this link before viewing the file to know if there are any additions/changes or corrections. The same link will be used for all of our work Physiology Edit 2 Mind map 3 COLON FUNCTIONS: Secretions of the Large Intestine: Mucus Secretion. • The mucosa of the large intestine has many crypts of 3 Colon consist of : Lieberkühn. • Absence of villi. • Ascending • Transverse • The epithelial cells contain almost no enzymes. • Descending • Presence of goblet cells that secrete mucus (provides an • Sigmoid adherent medium for holding fecal matter together). • Rectum • Anal canal • Stimulation of the pelvic nerves1 from the spinal cord can cause: Functions of the Large Intestine: o marked increase in mucus secretion. o This occurs along with increase in peristaltic motility 1. Reabsorb water and compact material of the colon. into feces. 2. Absorb vitamins produced by bacteria. • During extreme parasympathetic stimulation, so much 3. Store fecal matter prior to defecation. mucus can be secreted into the large intestine that the person has a bowel movement of ropy2 mucus as often as every 30 minutes; this mucus often contains little or no 1: considered a part of parasympathetic in large intestine . fecal material. 2: resembling a rope in being long, strong, and fibrous 3: anatomical division. 4 ILEOCECAL VALVE It prevents backflow of contents from colon into small intestine. -

Human Body- Digestive System
Previous reading: Human Body Digestive System (Organs, Location and Function) Science, Class-7th, Rishi Valley School Next reading: Cardiovascular system Content Slide #s 1) Overview of human digestive system................................... 3-4 2) Organs of human digestive system....................................... 5-7 3) Mouth, Pharynx and Esophagus.......................................... 10-14 4) Movement of food ................................................................ 15-17 5) The Stomach.......................................................................... 19-21 6) The Small Intestine ............................................................... 22-23 7) The Large Intestine ............................................................... 24-25 8) The Gut Flora ........................................................................ 27 9) Summary of Digestive System............................................... 28 10) Common Digestive Disorders ............................................... 31-34 How to go about this module 1) Have your note book with you. You will be required to guess or answer many questions. Explain your guess with reasoning. You are required to show the work when you return to RV. 2) Move sequentially from 1st slide to last slide. Do it at your pace. 3) Many slides would ask you to sketch the figures. – Draw them neatly in a fresh, unruled page. – Put the title of the page as the slide title. – Read the entire slide and try to understand. – Copy the green shade portions in the note book. 4) -

Normal Gastrointestinal Motility and Function Esophagus
Normal Gastrointestinal Motility and Function "Motility" is an unfamiliar word to many people; it is used primarily to describe the contraction of the muscles in the gastrointestinal tract. Because the gastrointestinal tract is a circular tube, when these muscles contract, they close off the tube or make the opening inside smaller - they squeeze. These muscles can contract in a synchronized way to move the food in one direction (usually downstream, but occasionally upstream for short distances); this is called peristalsis. If you looked at the intestine, you would see a ring of contraction that moves along pushing contents ahead of it. At other times, the muscles in adjacent parts of the gastrointestinal tract squeeze more or less independently of each other: this has the effect of mixing the contents but not moving them up or down. Both kinds of contraction patterns are called motility. The gastrointestinal tract is divided into four distinct parts: the esophagus, stomach, small intestine, and large intestine (colon). They are separated from each other by special muscles called sphincters which normally stay tightly closed and which regulate the movement of food and food residues from one part to another. Each part of the gastrointestinal tract has a unique function to perform in digestion, and as a result each part has a distinct type of motility and sensation. When motility or sensations are not appropriate for performing this function, they cause symptoms such as bloating, vomiting, constipation, or diarrhea which are associated with subjective sensations such as pain, bloating, fullness, and urgency to have a bowel movement. -

The Digestive System
THE DIGESTIVE SYSTEM COMPILED BY HOWIE BAUM DIGESTIVE SYSTEM People are probably more aware of their digestive system than of any other system, not least because of its frequent messages. Hunger, thirst, appetite, gas ☺, and the frequency and nature of bowel movements, are all issues affecting daily life. The Digestive Tract • Six Functions of the Digestive System 1. Ingestion 2. Mechanical processing 3. Digestion 4. Secretion 5. Absorption 6. Excretion The Digestive Tract • Ingestion – Occurs when materials enter digestive tract via the mouth • Mechanical Processing – Crushing and shearing – Makes materials easier to propel along digestive tract • Digestion – The chemical breakdown of food into small organic fragments for absorption by digestive epithelium The Digestive Tract • Secretion – Is the release of water, acids, enzymes, buffers, and salts – By epithelium of digestive tract – By glandular organs • Absorption – Movement of organic substrates, electrolytes, vitamins, and water – Across digestive epithelium tissue – Into the interstitial fluid of digestive tract • Excretion – Removal of waste products from body fluids – Process called defecation removes feces AN INTRODUCTION TO THE DIGESTIVE SYSTEM • The Digestive Tract • Also called the gastrointestinal (GI) tract or alimentary canal • Is a muscular tube • Extends from our mouth to the anus • Passes through the pharynx, esophagus, stomach, and small and large intestines The digestive system is one of the most clearly defined in the body. It consists of a long passageway, the digestive -
Appendix =(Sigmoid Pelvis)
MIND MAP ABDOMEN PELVIS PERINEUM Dr.Ahmed Kamal Large intestine [email protected] Abdomen Pelvis Perineum . Cecum . Sigmoid colon . Anal canal . Appendix =(Sigmoid pelvis) . Ascending colon . Rectum . Transverse colon . Descending colon (NOT FOUND IN RECTUM & ANAL CANAL) 1)Taeniae coli (3) longitudinal muscle bands. (Smooth muscles can be seen by naked eyes) 2)Sacculations Because the Taeniae coli are shorter than large intestine. (Haustra) 3)Epiploic Short peritoneal folds filled with fat. Appendices PARTS WITH MESENTERY RETROPERITONEAL PARTS PARTS DEVOID*OF CATS ADU PERITONEAL COVERING . Cecum . Ascending colon* . Lower 1/3 of rectum . Appendix . Descending colon . Anal canal . Transverse colon . Upper 2/3 of rectum . Sigmoid colon *Devoid= uncovered * Ascending colon thicker than Descending > the material inside it move against gravity ANTERIOR RELATION Anterior abdominal wall Greater omentum Coils of small intestine POSTERIOR RELATION Ascending Descending Cecum . Right kidney . Left kidney . Psoas major . Quadratus lumborum . Quadratus lumborum . Iliacus . Iliacus . Iliacus , Psoas major Anterior Posterior Superior Inferior . Anterior abdominal wall . 2nd part of duodenum . Liver Coils of small intestine . Greater omentum . Pancreas . Gall bladder . Superior mesenteric vessels. Stomach Left colic flexure is higher and more acute Hepatic flexure ( right colic flexure) Splenic flexure ( left colic flexure) Surface anatomy: the base of appendix is marked by McBurney’s point What is McBurney’s point ? A point at the junction of lateral 1/3 & medial 2/3 of a line traced from right anterior superior iliac spine to umbilicus. Opening: At posteromedial aspect of cecum, 1 inch below ileo-cecal junction Positions: 1.Retrocecal (most common) 2.Pelvic 3.Subcecal 4.Preilieal 5.Postileal ( least common) McBurney’s point Beginning Termination as a continuation continues as anal canal of sigmoid colon at level of S3. -

The Digestive System
Information about The Digestive System What is the Digestive System? The digestive system (gut) is a tube that goes from the mouth through the chest and abdomen to the back passage (anus). It is divided into several sections, each of which has a specialised function. Two other organs are closely involved in digestion, the liver and pancreas. They are attached to the gut by small tubes. These tubes carry the bile and enzymes made by the liver and pancreas to mix with food and break it into particles that can be absorbed. Food, fluid and waste products are pushed along the gut by muscular contractions in the wall which are called ‘peristalsis’. The time taken for food to go from the mouth to the anus varies from 12 to 48 hours, depending on the type of food eaten. The parts of the gut 1. The Mouth This is the beginning of the digestive process, where food is chewed and broken down into pieces that can be swallowed. Some people have difficulty chewing and swallowing food because of the poor state of their teeth or because they have a very dry mouth that doesn’t produce saliva. Some people have difficulty swallowing because of problems at the back of the mouth cavity, such as weakness of the swallowing muscles. 2. The Oesophagus The oesophagus is the tube that connects the mouth to the stomach. Muscle contractions in the oesophagus push food gently down into the stomach. There is a little valve between the oesophagus and the stomach called the ‘lower oesophageal valve.’ It prevents reflux (backwards movement) of acid and food back up into the oesophagus. -

Gas in the Digestive Tract
Gas in the Digestive Tract National Digestive Diseases Information Clearinghouse What is gas? Gas is air in the digestive tract—the large, muscular tube that extends from the mouth to the anus, where the movement of muscles, U.S. Department along with the release of hormones and Esophagus of Health and Mouth Human Services enzymes, allows for the digestion of food. Gas leaves the body when people burp through the NATIONAL mouth or pass gas through the anus. INSTITUTES Stomach OF HEALTH Gas is primarily composed of carbon dioxide, oxygen, nitrogen, hydrogen, and sometimes methane. Flatus, gas passed through the anus, may also contain small amounts of Large gasses that contain sulfur. Flatus that intestine contains more sulfur gasses has more odor. Everyone has gas. However, many people think they burp or pass gas too often and that Small Colon they have too much gas. Having too much intestine (shaded) gas is rare. Anus Rectum What causes gas? Gas in the digestive tract is usually caused The digestive tract by swallowing air and by the breakdown of certain foods in the large intestine by bacteria. Burping allows some gas to leave the stomach. The remaining gas moves into the Everyone swallows a small amount of air small intestine, where it is partially absorbed. when eating and drinking. The amount of air A small amount travels into the large swallowed increases when people intestine for release through the anus. • eat or drink too fast The stomach and small intestine do not • smoke fully digest some carbohydrates—sugars, starches, and fiber found in many foods. -
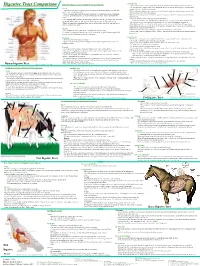
Digestive Tract Comparison • the Small Intestine Is a Tube Roughly Twenty Feet Long Deided Into the Duodenum, Jejunum and Ileum
• Small Intestine Human/Dog Digestive system or Simple Monogastric Digestion Digestive Tract Comparison • The small intestine is a tube roughly twenty feet long deided into the duodenum, jejunum and ileum. • The first part of the small intestine is the duodenum, the site of most chemical digestive reactions and is Mouth smoother than the rest of the small intestine • A specialized region of the digestive tract designed to break up large particles of food into • Bile, bicarbonate and pancreatic enzymes are secreted into the duodenum to breakdown nutrients in the smaller, more manageable particles chyme so that they can be readily absorbed. • Saliva is added to moisten food and begin carbohydrate breakdown by amylase in humans. •Bicarbonate from the pancreas neutralizes corrosive stomach acid from 3.5 in the stomach to 8.5 in the • There are four main types of teeth in the human or dog: incisors, canines, premolars and small intestine. molars. •Pancreatic enzymes include lipases, peptidases and amylases. •One reason dog and cat canines are much larger than ours is that they need to be able to rip and •Lipases break down fats. Peptidases break down proteins. Amylases break down carbohydrates. tear through tough raw meat. Humans have evolved to eat easier to chew, cooked meat. • Bile from the liver is stored in the gall bladder and secreted into the duodenum to emulsify fat. • While chewing, food is transformed into what is called a bolus, a food ball, and then forced •The jejunum and ileum are next in the small intestine and are covered in villi, finger-like projections. -

Healthy Digestion
SELINSGROVE AREA HIGH SCHOOL SEALS Health News VOLUME III, ISSUE 7 M A R C H 2 0 1 9 Healthy Digestion Good (healthy) digestion is a 'silent' process - digestion in some form is taking place while we rest, eat, sleep or work. We generally only be- come aware of digestion when something goes wrong (eg, if you eat foods that don't agree with your body or drink too much alcohol or say, if you become constipated or have gas). Although the digestive system can withstand a lot of stress (from the foods you eat to emotional stresses), it can only do so for a limited pe- riod. Over time, the negative effects will accumulate and create health problems in the long-term. So irrespective of your lifestyle in the past, you can take some positive steps today to rejuvenate and maintain the health of your digestive system. Maintaining a healthy digestive system - key points Eat a healthy diet Eat moderately, slowly and regularly Exercise regularly Reduce/manage stress levels Quit smoking P A G E 2 Wash your Your Digestive System & How it Works hands well af- What is the digestive system? ter using the bathroom, AND The digestive system is made up of the gastrointestinal tract—also called the leave your GI tract or digestive tract—and the liver, pancreas, and gallbladder. The GI tract is a series of hollow organs joined in a long, twisting tube from the phone out of mouth to the anus. The hollow organs that make up the GI tract are the the bathroom! mouth, esophagus, stomach, small intestine, large intestine, and anus. -

Anatomy of the Caecum, Appendix and Colon Is the Branches of the Middle and Left Colic Vessels, Resulting in Described
BASIC SCIENCE colon. The embryonic gut then twists to the right (ascending Anatomy of the caecum, colon) and then to the left (descending colon) so these parts become retroperitoneal. It drags its blood supply with it which appendix and colon explains why the right colon is supplied by branches of the superior mesenteric artery and the left colon by the inferior Harold Ellis mesenteric artery. Surgical mobilization of the colon follows these tissue planes to restore its midline position, thus the safe approach on each side is from lateral to medial. There is Abstract a natural vascular watershed in the transverse colon between The gross and microscopic anatomy of the caecum, appendix and colon is the branches of the middle and left colic vessels, resulting in described. An embryological explanation of the adult form is included. the splenic flexure being particularly vulnerable to ischaemia. There is also a note on cancer spread. Peritoneal attachments Keywords Anatomy; appendix; ascending colon; blood supply; caecum; The transverse and sigmoid colon are completely peritonealized, descending colon; lymphatic drainage; sigmoid colon; transverse colon hanging onto the transverse and the sigmoid mesocolon respec- tively. The transverse colon is readily identified by its attachment, along its free border, to the greater omentum. In contrast, the ascending and descending colons adhere to the peritoneum of the The large bowel is subdivided for descriptive purposes into: the posterior abdominal wall. This adhesion is avascular, and enables caecum and appendix, the ascending colon, hepatic flexure, the surgeon easily to mobilize these parts of the large bowel. The transverse colon, splenic flexure, descending and sigmoid colon caecum is usually completely peritonealized, as may occasionally and the rectum and anal canal (Figure 1).