Opioid-Induced Constipation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Laxatives for the Management of Constipation in People Receiving Palliative Care (Review)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UCL Discovery Laxatives for the management of constipation in people receiving palliative care (Review) Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2015, Issue 5 http://www.thecochranelibrary.com Laxatives for the management of constipation in people receiving palliative care (Review) Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 BACKGROUND .................................... 2 OBJECTIVES ..................................... 4 METHODS ...................................... 4 RESULTS....................................... 7 Figure1. ..................................... 8 Figure2. ..................................... 9 Figure3. ..................................... 10 DISCUSSION ..................................... 13 AUTHORS’CONCLUSIONS . 14 ACKNOWLEDGEMENTS . 14 REFERENCES ..................................... 15 CHARACTERISTICSOFSTUDIES . 17 DATAANDANALYSES. 26 ADDITIONALTABLES. 26 APPENDICES ..................................... 28 WHAT’SNEW..................................... 35 HISTORY....................................... 35 CONTRIBUTIONSOFAUTHORS . 36 DECLARATIONSOFINTEREST . 36 SOURCESOFSUPPORT . 36 DIFFERENCES -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -
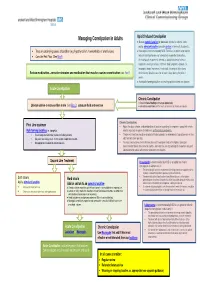
Managing Constipation in Adults
Managing Constipation in Adults Opio id Induced Constipation ● Use an osmotic laxative (or docusate which also softens stools and a stimulant laxative (consider dantron in terminally ill patients). • Treat any underlying causes of condition (e.g. hypothyroidism, haemorrhoids or anal fissures) ● Naloxegol is recommended by NICE TA345 as an option when opioid induced constipation has not adequately responded to laxatives. • Consider Red Flags (See Box A ) An inadequate response is defined as opioid-induced of at least moderate severity in at least 1of the 4 stool symptoms domain (i.e. incomplete bowel movement, hard stool, straining or false alarm) Review medication –consider stopping any medication that may be causing constipation (see Box B ) whilst taking1 laxative class for at least 4 days during the prior 2 weeks. ● Avoid bulk -forming l axatives for treating opioid induced constipation Acute Constipation Chronic Constipation ● Check for faecal loading and manage appropriately Lifestyle advise -to increase fibre in diet (see Box C ), adequate fluid and exercise ● Set realistic expectations for the results of treatment of chronic constipation. Chronic Constipation: First Line treatment • Adjust the dose, choice, and combination of laxative according to symptoms, speed with which Bulk forming laxatives i.e. ispaghula. relief is required, response to treatment, and individual preference. • Ensure adequate fluid intake (caution- frail elderly patients) • The dose of laxative should be gradually titrated upwards (or downwards) to produce one or two • May take several days to act- so not suitable if rapid relief required. soft, formed stools per day. • Not appropriate for opiod induced constipation. • If at least two laxatives (from different classes) have been tried at the highest tolerated recommended doses for at least 6 months, consider the use of prucalopride in women only and lubiprostone for adults with chronic idiopathic constipation. -

COMPASS Therapeutic Notes on the Management of Chronic Constipation in Primary Care
COMPASS Therapeutic Notes on the Management of Chronic Constipation in Primary Care In this issue: Glossary Page Chronic Constipation lasting longer than 3 months Introduction and background 1 constipation Drugs used in the management of Functional Chronic constipation without a known cause. Also known as primary 3 chronic constipation constipation constipation or idiopathic constipation Bulk-forming laxatives 4 Gastrocolic The occurrence of peristalsis following the entrance of food into the Osmotic laxatives 5 response empty stomach Stimulant laxatives 6 IBS Irritable Bowel Syndrome IBS-C Irritable Bowel Syndrome with Constipation Faecal softeners 7 Melanosis Dark brownish black pigmentation of the mucous membrane of the colon Peripheral opioid-receptor 7 coli due to the deposition of pigment in macrophages antagonists Myenteric Part of the enteric nervous system with an important role in regulating 5HT4 – receptor agonists 7 plexus gut motility Managing constipation in 8 NNT Number Needed to Treat pregnancy and breastfeeding OTC Over The Counter RCT Randomised Controlled Trial Secondary Constipation caused by a drug or medical condition. Secondary constipation constipation is also known as organic constipation SmPC Summary of Product Characteristics A twisting or looping of the bowel resulting in obstruction; can be life- Volvulus threatening Successful completion of the assessment questions at the end of this issue will provide you with 2 hours towards your CPD/CME requirements. Further copies of this and any other edition in the -

1: Gastro-Intestinal System
1 1: GASTRO-INTESTINAL SYSTEM Antacids .......................................................... 1 Stimulant laxatives ...................................46 Compound alginate products .................. 3 Docuate sodium .......................................49 Simeticone ................................................... 4 Lactulose ....................................................50 Antimuscarinics .......................................... 5 Macrogols (polyethylene glycols) ..........51 Glycopyrronium .......................................13 Magnesium salts ........................................53 Hyoscine butylbromide ...........................16 Rectal products for constipation ..........55 Hyoscine hydrobromide .........................19 Products for haemorrhoids .................56 Propantheline ............................................21 Pancreatin ...................................................58 Orphenadrine ...........................................23 Prokinetics ..................................................24 Quick Clinical Guides: H2-receptor antagonists .......................27 Death rattle (noisy rattling breathing) 12 Proton pump inhibitors ........................30 Opioid-induced constipation .................42 Loperamide ................................................35 Bowel management in paraplegia Laxatives ......................................................38 and tetraplegia .....................................44 Ispaghula (Psyllium husk) ........................45 ANTACIDS Indications: -

National Institute for Clinical Excellence
Appendix C NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Single Technology Appraisal (STA) Lubiprostone for treating opioid-induced constipation in people with chronic, non-cancer pain Matrix of consultees and commentators Consultees Commentators (no right to submit or appeal) Manufacturers/sponsors General Sucampo Pharma Europe Allied Health Professionals Federation (lubiprostone) Board of Community Health Councils in Wales Patient/carer groups British National Formulary Action on Pain Care Quality Commission Afiya Trust Commissioning Support Appraisals Black Health Agency Service Bladder and Bowel Foundation Department of Health, Social Services Equalities National Council and Public Safety for Northern Ireland Muslim Council of Britain Healthcare Improvement Scotland Muslim Health Network Medicines and Healthcare products Pain Concern Regulatory Agency Pain Relief Foundation National Association of Primary Care Pain UK National Pharmacy Association PromoCon NHS Alliance South Asian Health Foundation NHS Commercial Medicines Unit Specialised Healthcare Alliance NHS Confederation IBS Network Public Health Wales NHS Trust Scottish Medicines Consortium Professional groups Association of Coloproctology of Great Comparator manufacturers Britain and Ireland Abbott Laboratories UK (lactulose) Association for Continence Advice Actavis UK (glycerol suppositories), Association for Palliative Medicine Amdipharm (methylcellulose) British Geriatrics Society Bell Sons & Co (Druggists) Limited British Pain -

A Four-Country Comparison of Healthcare Systems, Implementation
Neurogastroenterology & Motility Neurogastroenterol Motil (2014) 26, 1368–1385 doi: 10.1111/nmo.12402 REVIEW ARTICLE A four-country comparison of healthcare systems, implementation of diagnostic criteria, and treatment availability for functional gastrointestinal disorders A report of the Rome Foundation Working Team on cross-cultural, multinational research M. SCHMULSON,* E. CORAZZIARI,† U. C. GHOSHAL,‡ S.-J. MYUNG,§ C. D. GERSON,¶ E. M. M. QUIGLEY,** K.-A. GWEE†† & A. D. SPERBER‡‡ *Laboratorio de Hıgado, Pancreas y Motilidad (HIPAM)-Department of Experimental Medicine, Faculty of Medicine-Universidad Nacional Autonoma de Mexico (UNAM). Hospital General de Mexico, Mexico City, Mexico †Gastroenterologia A, Department of Internal Medicine and Medical Specialties, University La Sapienza, Rome, Italy ‡Department of Gastroenterology, Sanjay Gandhi Postgraduate Institute of Medical Science, Lucknow, India §Department of Gastroenterology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea ¶Division of Gastroenterology, Mount Sinai School of Medicine, New York, NY, USA **Division of Gastroenterology and Hepatology, Houston Methodist Hospital and Weill Cornell Medical College, Houston, TX, USA ††Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore ‡‡Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer-Sheva, Israel Key Messages • This report identified seven key issues related to healthcare provision that may impact how patients with FGIDs are investigated, diagnosed and managed. • Variations in healthcare provision around the world in patients with FGIDs have not been reviewed. • We compared four countries that are geographically and culturally diverse, and exhibit differences in the healthcare coverage provided to their population: Italy, South Korea, India and Mexico. • Since there is a paucity of publications relating to the issues covered in this report, some of the findings are based on the authors’ personal perspectives, press reports and other published sources. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Summary of Product Characteristics
Irish Medicines Board Summary of Product Characteristics 1 NAME OF THE MEDICINAL PRODUCT Codalax Forte 1000mg/75mg per 5ml suspension 2 QUALITATIVE AND QUANTITATIVE COMPOSITION 5ml of suspension contains 1000mg Poloxmer 188 and 75mg Dantron. Excipients: Each 5ml also contain: 1000mg Sorbitol liquid (non -crystallising), 0.2ml 96% ethanol and 10mg Niasept sodium containing sodium ethyl, methyl and propyl parahydroxybenzoate (E 215, E 219 and E 217) For a full list of excipients, see section 6.1 3 PHARMACEUTICAL FORM Oral suspension. Orange -yellow coloured oral suspension, with an odour of peach and a sweet taste. 4 CLINICAL PARTICULARS 4.1 Therapeutic Indications Use only in the treatment of analgesic induced constipation in the terminally ill patient. 4.2 Posology and method of administration Oral Adults and Elderly One 5 ml spoonfuls at bedtime. Children Not recommended for children under twelve years of age. 4.3 Contraindications ○ In common with other gastro -intestinal evacuants, CODALAX and CODALAX Forte suspensions should not be given when acute or painful conditions of the abdomen are present or when the cause of constipation is suspected to be intestinal obstruction. ○ Pregnancy. ○ Hypersensitivity to any of the constituents of the products. 4.4 Special warnings and precautions for use ○ Oral administration of dantron has been reported to cause intestinal tumours in rats and mice. It has also been reported to be hepatocarcinogenic in rats as well as in mice. There is no sound evidence to conclude a no effect dose and therefore there may be a risk of such effects in humans. ○ In babies, children and patients wearing nappies, there may be staining of the buttocks. -

Laxatives Or Methylnaltrexone for the Management of Constipation in Palliative Care Patients (Review)
Laxatives or methylnaltrexone for the management of constipation in palliative care patients (Review) Candy B, Jones L, Goodman ML, Drake R, Tookman A This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2011, Issue 1 http://www.thecochranelibrary.com Laxatives or methylnaltrexone for the management of constipation in palliative care patients (Review) Copyright © 2011 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 SUMMARY OF FINDINGS FOR THE MAIN COMPARISON . ..... 3 BACKGROUND .................................... 4 OBJECTIVES ..................................... 4 METHODS...................................... 5 RESULTS....................................... 7 Figure1. ..................................... 8 Figure2. ..................................... 9 DISCUSSION ..................................... 13 AUTHORS’CONCLUSIONS . 14 ACKNOWLEDGEMENTS . 15 REFERENCES..................................... 15 CHARACTERISTICSOFSTUDIES . .. 17 DATAANDANALYSES. 28 Analysis 1.1. Comparison 1 Methylnaltrexone versus placebo, Outcome 1 Proportion who had rescue-free laxation within 4hours..................................... 28 Analysis 1.2. Comparison 1 Methylnaltrexone versus placebo, Outcome 2 Laxation within 24 hours. 29 Analysis 1.3. Comparison 1 Methylnaltrexone versus placebo, Outcome 3 Tolerability: proportion -

Chemical and Herbal Remedies For
Indian Journal of Drugs, 2013, 1(2), 23-37 ISSN: 2348-1684 CHEMICAL AND HERBAL REMEDIES FOR CONSTIPATED PATIENTS: A REVIEW Neeraj Kumar1* and Kamal Kishore2 1Assistant Professor, Shri Ram Murti Smarak College of Engineering and Technology (Pharmacy), Nainital road, Bareilly- 243202, U.P. (India). 2Associate Professor, Department of Pharmacy, M. J. P. Rohilkhand University, Bareilly-243006, U.P. (India). *For Correspondence: Abstract Corresponding author email: Constipation is a condition in which the feces are dry and hard [email protected], Mob: +919897325740 with infrequent difficult evacuation. In 5-25 % children and 2% of Received: 26.08.2013 total population experiences great difficulty with elimination of Accepted: 22.12.2013 food waste, accompanied with pain, fear, and avoidance, treated Access this article online by laxatives, the agents that add bulk to intestinal contents or Website: stimulation of intestinal secretion or motility. The foods like www.drugresearch.in prunes, pears, bran cereals, chemicals like salts of magnesium, Quick Response Code: cellulose, plants like senna, encourage intestinal contractions and easier time moving of bowels. This systematic review contains a huge collection of references for chemicals, natural products, plants having laxative action and also provides the information about medical conditions and diseases responsible for constipation. Keywords: Constipation, laxative, stimulants, transit time, defecation. INTRODUCTION axatives are the agents that increase committee and American college of the ease and frequency of defecation gastroenterology chronic constipation task Lby adding bulk to intestinal contents by force specify that symptoms must have retaining the water in bowel or by occurred for at least 12 weeks in the increasing motility or by stimulating preceding of 12 months (Hsieh, 2005). -

1. Gastro-Intestinal System
1 1. Gastro-intestinal system Also see Appendix 1B Guidance on Management of Dyspepsia http://www.fifeadtc.scot.nhs.uk/formulary/1-gastro-intestinal/appendix-1b-management-of- dyspepsia.aspx Also see SIGN 68 – Dyspepsia, March 2003 www.sign.ac.uk/guidelines/fulltext/68/index.html. Also see NICE CG17 - Dyspepsia – Management of Dyspepsia in Adults in Primary Care, August 2004 http://www.nice.org.uk/guidance/CG17 1.1 - Dyspepsia and gastro-oesophageal reflux disease 1.1.1 Antacids and simeticone Aluminium- and magnesium-containing antacids Co-magaldrox (Mucogel®) Simeticone alone Infacol® Prescribing Points Lifestyle changes are often required, such as raising the head of the bed, weight reduction, reduction of alcohol, smoking cessation and avoidance of aggravating foods. Antacids are suitable for mild indigestion and reflux. A mixture of aluminium hydroxide and magnesium hydroxide balances the tendency of aluminium to constipate against that of magnesium to cause diarrhoea. Liquid preparations are normally more effective than tablets and quicker acting. Antacids, taken at the same time as other drugs, may impair their absorption. They may also damage enteric coatings designed to prevent irritant drugs from dissolving in the stomach Mucogel® has a low Na+ content (less than 1mmol of Na+ per tablet/10ml) and is sugar-free. There is no evidence that simeticone containing products like Infacol® are any more effective than placebo in reducing colic episodes. An unlicensed mixture containing antacid + oxetacaine (previously marketed as Mucaine®) is approved for use in patients presenting with mucositis after treatment with radiotherapy. 1.1.2 Compound alginates and proprietary indigestion preparations 1st Choice Acidex® 2nd Choice Gaviscon Advance® Child Gaviscon Infant® sachets Prescribing Points Compound alginate preparations contain multiple ingredients; prescribe by brand name to avoid any confusion.