Managing Constipation in Older People
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

COMFORCLEANSE Item No
COMFORCLEANSE Item No. 320402 Size: 150 ct AUST L 193422 VEGAN PRODUCT SUMMARY & BACKGROUND ComforCleanse™ is an effective combination of herbs and essential oils that aids in the relief of constipation and helps reduce the occurrence of constipation. It effectively combines healthy fibre, minerals, herbs and essential oils to cleanse and balance. Conveniently packaged in capsule form, ComforCleanse also contains Cascara sagrada bark and psyllium. The colon is the final component of the digestive tract; it extracts water and salt from solid wastes before they are eliminated from the body. Although the bowel is comprised of the small and the large intestines, it is commonly used to describe the large intestines, which consist of the colon, the rectum and the anus. Therefore, a ‘bowel problem’ is any problem affecting these parts of the digestive tract. ComforCleanse was formulated to help cleanse and restore balance with the entire bowel in mind. KEY INGREDIENTS Berberis vulgaris, Burdock Root Powder, Chamomile Mentha piperita, pectin, Pimpinella anisum, Psyllium Oil German, Citrus reticulata, Copaiba Oil, Husk Powder, Rheum palmatum, Rosmarinus Echinacea purpurea, Ferula assa-foetida, Foeniculum officinalis, Zingiber officinale vulgare, Frangula purshiana, Garlic Clove Powder, FEATURES BENEFITS • Vegan-friendly formula • Decrease and relieve constipation • Contains Cascara sagrada (Frangula purshiana) • Can be used as a laxative bark traditionally used as a stimulant laxative • Helps reduce occurrence of constipation • Promotes bowel evacuation • Improves bowel waste elimination • Supports normal digestive and colon function WHO SHOULD TAKE COMFORCLEANSE? DIRECTIONS Those suffering from the uncomfortable effects of Adults take 2-3 capsules before breakfast and at constipation bedtime. Drink at least 2L of water throughout the day for best results. -
Final Stakeholder List PDF 190 KB
Appendix C NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Single Technology Appraisal Naldemedine for treating opioid-induced constipation [ID1189] Matrix of consultees and commentators Consultees Commentators (no right to submit or appeal) Company General • Shionogi (naldemedine) • All Wales Therapeutics and Toxicology Centre Patient/carer groups • Allied Health Professionals Federation • Action on Pain • Board of Community Health Councils in • Black Health Agency Wales • Bladder and Bowel Community • British National Formulary • Bladder and Bowel UK • Care Quality Commission • Cancer Black Care • Department of Health, Social Services • Cancer Equality and Public Safety for Northern Ireland • Guts UK • Healthcare Improvement Scotland • HAWC • Medicines and Healthcare products • Helen Rollason Cancer Charity Regulatory Agency • IBS Network • National Association of Primary Care • Independent Cancer Patients Voice • National Pharmacy Association • Macmillan Cancer Support • NHS Alliance • Maggie’s Centres • NHS Confederation • Marie Curie • Scottish Medicines Consortium • Muslim Council of Britain • Scottish Society of Gastroenterology • Pain Concern • Welsh Health Specialised Services • Pain Relief Foundation Committee • Pain UK • South Asian Health Foundation Possible comparator companies • Specialised Healthcare Alliance • Actavis UK (glycerol suppositories, • Tenovus Cancer Care bisacodyl, lactulose, senna) • Wellbeing of Women • Cardinal Health Martindale Products • Women’s Health Concern (glycerol suppositories) • Concordia International -

G.I. Fortify (Capsules) Introduced 2013
G.I. Fortify (capsules) Introduced 2013 What Is It? Recommendations G.I. Fortify (capsules) is designed to support G.I. health and bowel regularity as well Pure Encapsulations recommends 3-6 capsules daily, in divided doses, with a meal as promote a healthy G.I. environment, detoxification and colon cell function.* and 8-12 oz. water. Uses For G.I. Fortify (capsules) Are There Any Potential Side Effects Or Precautions? G.I. Motility: Psyllium, Plantago indica or blond psyllium, is a valued source of Not to be taken by pregnant or lactating women. Psyllium and flaxseed may cause soluble fiber. Soluble fiber increases stool volume when taken with appropriate gastrointestinal discomfort, including bloating, flatulence, addominal pain or amounts of water, supporting larger and softer stools for healthy bowel diarrhea. In rare cases, psyllium has been associated with headache, backache, movements. As the bulk moves through the intestine, it helps to collect and rhinitis, increased cough, and sinusitis. Psyllium should be consumed with eliminate other waste and toxins from the intestinal walls. This helps to minimize adequate water, as case reports indicate a potential for bowel obstruction when the amount of exposure of the gastrointestinal tract to toxins. Flaxseeds provide it is consumed without water. Rarely, individuals can have an allergic response a source of lignans, fatty acids, and both soluble and insoluble fibers, enhancing to psyllium, with symptoms including runny nose, sneezing, conjunctivitis, skin the gut health potential of this complex.* rash, itching, flushing, chest and throat tightness, congestion, hypotension or G.I. Integrity: l-Glutamine is the most abundant amino acid in the body. -

Laxatives for the Management of Constipation in People Receiving Palliative Care (Review)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UCL Discovery Laxatives for the management of constipation in people receiving palliative care (Review) Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2015, Issue 5 http://www.thecochranelibrary.com Laxatives for the management of constipation in people receiving palliative care (Review) Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 BACKGROUND .................................... 2 OBJECTIVES ..................................... 4 METHODS ...................................... 4 RESULTS....................................... 7 Figure1. ..................................... 8 Figure2. ..................................... 9 Figure3. ..................................... 10 DISCUSSION ..................................... 13 AUTHORS’CONCLUSIONS . 14 ACKNOWLEDGEMENTS . 14 REFERENCES ..................................... 15 CHARACTERISTICSOFSTUDIES . 17 DATAANDANALYSES. 26 ADDITIONALTABLES. 26 APPENDICES ..................................... 28 WHAT’SNEW..................................... 35 HISTORY....................................... 35 CONTRIBUTIONSOFAUTHORS . 36 DECLARATIONSOFINTEREST . 36 SOURCESOFSUPPORT . 36 DIFFERENCES -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -
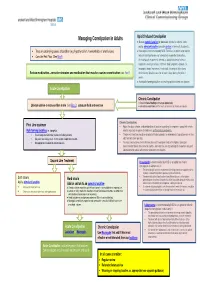
Managing Constipation in Adults
Managing Constipation in Adults Opio id Induced Constipation ● Use an osmotic laxative (or docusate which also softens stools and a stimulant laxative (consider dantron in terminally ill patients). • Treat any underlying causes of condition (e.g. hypothyroidism, haemorrhoids or anal fissures) ● Naloxegol is recommended by NICE TA345 as an option when opioid induced constipation has not adequately responded to laxatives. • Consider Red Flags (See Box A ) An inadequate response is defined as opioid-induced of at least moderate severity in at least 1of the 4 stool symptoms domain (i.e. incomplete bowel movement, hard stool, straining or false alarm) Review medication –consider stopping any medication that may be causing constipation (see Box B ) whilst taking1 laxative class for at least 4 days during the prior 2 weeks. ● Avoid bulk -forming l axatives for treating opioid induced constipation Acute Constipation Chronic Constipation ● Check for faecal loading and manage appropriately Lifestyle advise -to increase fibre in diet (see Box C ), adequate fluid and exercise ● Set realistic expectations for the results of treatment of chronic constipation. Chronic Constipation: First Line treatment • Adjust the dose, choice, and combination of laxative according to symptoms, speed with which Bulk forming laxatives i.e. ispaghula. relief is required, response to treatment, and individual preference. • Ensure adequate fluid intake (caution- frail elderly patients) • The dose of laxative should be gradually titrated upwards (or downwards) to produce one or two • May take several days to act- so not suitable if rapid relief required. soft, formed stools per day. • Not appropriate for opiod induced constipation. • If at least two laxatives (from different classes) have been tried at the highest tolerated recommended doses for at least 6 months, consider the use of prucalopride in women only and lubiprostone for adults with chronic idiopathic constipation. -

Cleanse & Lean
Max Cleanse & Lean Get Lean and Hard! † Lose Weight & Cleanse Your Body!† The research team at Max Muscle has come up with a product that not Size: 100 Capsules only improves your health, but also helps you lose excess weight fast! We Serving Size: 1 Capsule call it Max Cleanse & Lean™ (MCL). MCL offers you a solution to your Servings Per Container: Approx. 100 digestive issues as well as a way to remove excess water and waste from your body. You workout hard to build your body and you want to be able to KEY FEATURES • Get Lean & Hard † see the results every day, that’s what MCL can do. Often stress, poor diet, • Removes Excess Water† and every day issues affect our body’s ability to deal with excess fluid and • Promotes Regularity† waste causing numerous problems. MCL addresses the bloating or excess water that many of us hold just under our skin, in our hips, abs, legs, and KEY MESSAGES feet. MCL helps you naturally release this excess fluid and flush it from • Improves health and promotes quick weight-loss.† your system helping you drop weight and lean up. MCL also helps • Removes subcutaneous water and waste from the body.† promote regularity. Regularity is essential for your health. If you’re backed • Flushes excess fluid from the body.† up then MCL will help you get back on track and, in the process, enable • Promotes regularity.† your body to absorb more nutrients, grow more muscle, and lose • Formulated with the highest quality ingredients more weight.† to help you lean out and look better than ever.† • Patent pending formula designed to help you lose Patent Pending Formula! weight fast while cleansing system of impurities for † MCL was formulated with the highest quality ingredients available to help better health. -

The Heart Health Benefits of Dietary Fiber
Conclusions. Heart disease continues to be one of the most factors for cardiovascular disease, including blood pressure, weight, widespread health problems in the United States. Fortunately, it is and glucose levels. Not all fibers provide the same cardiovascular ™ also among the most preventable health problems. To that end, benefits; however, and differences among the various types of fibers Expert Views Americans are encouraged to adopt heart-healthy habits, which should be appreciated when choosing a fiber supplement. Psyllium include a healthy diet and regular exercise.7 In addition to being an and oat fibers are the only fibers that have been recognized by the GI HealtH & Wellness | Issue four | february 2011 important part of a healthy diet, dietary fibers provide a number of FDA for their cholesterol-lowering and cardiovascular benefits, while cardiovascular benefits. The cholesterol-lowering benefits of certain other fibers such as calcium polycarbophil are indicated for their fibers (psyllium and oat fibers) as adjunct to a low-fat diet are well- laxation effects. An understanding of these differences should allow features: the heart health benefits of recognized and have been demonstrated in numerous well-controlled physicians and patients to tailor their choice of dietary fiber and fiber trials. Further, dietary fibers have beneficial effects on other key risk supplements to better meet their individual health goals. One dietary fiber CHD Prevalence february Is amerIcan Heart HealtH montH Cholesterol Lowering Benefits Heart -

Various Species, Mainly Aloe Ferox Miller and Its Hybrids)
European Medicines Agency Evaluation of Medicines for Human Use London, 5 July 2007 Doc. Ref: EMEA/HMPC/76313/2006 COMMITTEE ON HERBAL MEDICINAL PRODUCTS (HMPC) ASSESSMENT REPORT ON ALOE BARABADENSIS MILLER AND ALOE (VARIOUS SPECIES, MAINLY ALOE FEROX MILLER AND ITS HYBRIDS) Aloe barbadensis Miller (barbados aloes) Herbal substance Aloe [various species, mainly Aloe ferox Miller and its hybrids] (cape aloes) the concentrated and dried juice of the leaves, Herbal Preparation standardised; standardised herbal preparations thereof Pharmaceutical forms Herbal substance for oral preparation Rapporteur Dr C. Werner Assessor Dr. B. Merz Superseded 7 Westferry Circus, Canary Wharf, London, E14 4HB, UK Tel. (44-20) 74 18 84 00 Fax (44-20) 75 23 70 51 E-mail: [email protected] http://www.emea.europa.eu ©EMEA 2007 Reproduction and/or distribution of this document is authorised for non commercial purposes only provided the EMEA is acknowledged TABLE OF CONTENTS I. Introduction 3 II. Clinical Pharmacology 3 II.1 Pharmacokinetics 3 II.1.1 Phytochemical characterisation 3 II.1.2 Absorption, metabolism and excretion 4 II.1.3 Progress of action 5 II.2 Pharmacodynamics 5 II.2.1 Mode of action 5 • Laxative effect 5 • Other effects 7 II.2.2 Interactions 8 III. Clinical Efficacy 9 III.1 Dosage 9 III.2 Clinical studies 9 Conclusion 10 III.3 Clinical studies in special populations 10 III.3.1 Use in children 10 III.3.2. Use during pregnancy and lactation 10 III.3.3. Conclusion 13 III.4 Traditional use 13 IV. Safety 14 IV.1 Genotoxic and carcinogenic risk 14 IV.1.1 Preclinical Data 14 IV.1.2 Clinical Data 18 IV.1.3 Conclusion 20 IV.2 Toxicity 20 IV.3 Contraindications 21 IV.4 Special warnings and precautions for use 21 IV.5 Undesirable effects 22 IV.6 Interactions 22 IV.7 Overdose 23 V. -

COMPASS Therapeutic Notes on the Management of Chronic Constipation in Primary Care
COMPASS Therapeutic Notes on the Management of Chronic Constipation in Primary Care In this issue: Glossary Page Chronic Constipation lasting longer than 3 months Introduction and background 1 constipation Drugs used in the management of Functional Chronic constipation without a known cause. Also known as primary 3 chronic constipation constipation constipation or idiopathic constipation Bulk-forming laxatives 4 Gastrocolic The occurrence of peristalsis following the entrance of food into the Osmotic laxatives 5 response empty stomach Stimulant laxatives 6 IBS Irritable Bowel Syndrome IBS-C Irritable Bowel Syndrome with Constipation Faecal softeners 7 Melanosis Dark brownish black pigmentation of the mucous membrane of the colon Peripheral opioid-receptor 7 coli due to the deposition of pigment in macrophages antagonists Myenteric Part of the enteric nervous system with an important role in regulating 5HT4 – receptor agonists 7 plexus gut motility Managing constipation in 8 NNT Number Needed to Treat pregnancy and breastfeeding OTC Over The Counter RCT Randomised Controlled Trial Secondary Constipation caused by a drug or medical condition. Secondary constipation constipation is also known as organic constipation SmPC Summary of Product Characteristics A twisting or looping of the bowel resulting in obstruction; can be life- Volvulus threatening Successful completion of the assessment questions at the end of this issue will provide you with 2 hours towards your CPD/CME requirements. Further copies of this and any other edition in the -

1: Gastro-Intestinal System
1 1: GASTRO-INTESTINAL SYSTEM Antacids .......................................................... 1 Stimulant laxatives ...................................46 Compound alginate products .................. 3 Docuate sodium .......................................49 Simeticone ................................................... 4 Lactulose ....................................................50 Antimuscarinics .......................................... 5 Macrogols (polyethylene glycols) ..........51 Glycopyrronium .......................................13 Magnesium salts ........................................53 Hyoscine butylbromide ...........................16 Rectal products for constipation ..........55 Hyoscine hydrobromide .........................19 Products for haemorrhoids .................56 Propantheline ............................................21 Pancreatin ...................................................58 Orphenadrine ...........................................23 Prokinetics ..................................................24 Quick Clinical Guides: H2-receptor antagonists .......................27 Death rattle (noisy rattling breathing) 12 Proton pump inhibitors ........................30 Opioid-induced constipation .................42 Loperamide ................................................35 Bowel management in paraplegia Laxatives ......................................................38 and tetraplegia .....................................44 Ispaghula (Psyllium husk) ........................45 ANTACIDS Indications: -

Immediate Hypersensitivity Reactions Caused by Drug Excipients: a Literature Review Caballero ML, Quirce S
REVIEWS Immediate Hypersensitivity Reactions Caused by Drug Excipients: A Literature Review Caballero ML, Quirce S Department of Allergy, La Paz University Hospital, IdiPAZ, Madrid, Spain J Investig Allergol Clin Immunol 2020; Vol. 30(2): 86-100 doi: 10.18176/jiaci.0476 Abstract The European Medicines Agency defines excipients as the constituents of a pharmaceutical form apart from the active substance. Immediate hypersensitivity reactions (IHRs) caused by excipients contained in the formulation of medications have been described. However, there are no data on the prevalence of IHRs due to drug excipients. Clinical manifestations of allergy to excipients can range from skin disorders to life-threatening systemic reactions. The aim of this study was to review the literature on allergy to pharmaceutical excipients and to record the IHRs described with various types of medications, specifically reactions due to the excipients contained in their formulations. The cases reported were sorted alphabetically by type of medication and excipient in order to obtain a list of the excipients most frequently involved for each type of medication. Key words: Allergy. Drug immediate hypersensitivity reaction. Excipient. Pharmaceutical excipients. Resumen La Agencia Europea de Medicamentos define los excipientes como los componentes de una forma farmacéutica diferenciados del principio activo. Se han descrito reacciones de hipersensibilidad inmediata causadas por los excipientes contenidos en la formulación de medicamentos. Sin embargo, no hay datos sobre la prevalencia de dichas reacciones. Las manifestaciones clínicas de la alergia a los excipientes pueden ir desde trastornos de la piel hasta reacciones sistémicas que ponen en peligro la vida. El objetivo de este estudio fue realizar una revisión de la literatura sobre la alergia a los excipientes farmacéuticos y recopilar las reacciones inmediatas descritas con diferentes tipos de medicamento, debido solo a excipientes contenidos en sus formulaciones.