Chemical and Herbal Remedies For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Classification of Medicinal Drugs and Driving: Co-Ordination and Synthesis Report
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Due date of deliverable: 21.07.2011 Actual submission date: 21.07.2011 Revision date: 21.07.2011 Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UVA Revision 0.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public PP Restricted to other programme participants (including the Commission x Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) DRUID 6th Framework Programme Deliverable D.4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Page 1 of 243 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Authors Trinidad Gómez-Talegón, Inmaculada Fierro, M. Carmen Del Río, F. Javier Álvarez (UVa, University of Valladolid, Spain) Partners - Silvia Ravera, Susana Monteiro, Han de Gier (RUGPha, University of Groningen, the Netherlands) - Gertrude Van der Linden, Sara-Ann Legrand, Kristof Pil, Alain Verstraete (UGent, Ghent University, Belgium) - Michel Mallaret, Charles Mercier-Guyon, Isabelle Mercier-Guyon (UGren, University of Grenoble, Centre Regional de Pharmacovigilance, France) - Katerina Touliou (CERT-HIT, Centre for Research and Technology Hellas, Greece) - Michael Hei βing (BASt, Bundesanstalt für Straßenwesen, Germany). -

Laxatives for the Management of Constipation in People Receiving Palliative Care (Review)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by UCL Discovery Laxatives for the management of constipation in people receiving palliative care (Review) Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2015, Issue 5 http://www.thecochranelibrary.com Laxatives for the management of constipation in people receiving palliative care (Review) Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 BACKGROUND .................................... 2 OBJECTIVES ..................................... 4 METHODS ...................................... 4 RESULTS....................................... 7 Figure1. ..................................... 8 Figure2. ..................................... 9 Figure3. ..................................... 10 DISCUSSION ..................................... 13 AUTHORS’CONCLUSIONS . 14 ACKNOWLEDGEMENTS . 14 REFERENCES ..................................... 15 CHARACTERISTICSOFSTUDIES . 17 DATAANDANALYSES. 26 ADDITIONALTABLES. 26 APPENDICES ..................................... 28 WHAT’SNEW..................................... 35 HISTORY....................................... 35 CONTRIBUTIONSOFAUTHORS . 36 DECLARATIONSOFINTEREST . 36 SOURCESOFSUPPORT . 36 DIFFERENCES -

NINDS Custom Collection II
ACACETIN ACEBUTOLOL HYDROCHLORIDE ACECLIDINE HYDROCHLORIDE ACEMETACIN ACETAMINOPHEN ACETAMINOSALOL ACETANILIDE ACETARSOL ACETAZOLAMIDE ACETOHYDROXAMIC ACID ACETRIAZOIC ACID ACETYL TYROSINE ETHYL ESTER ACETYLCARNITINE ACETYLCHOLINE ACETYLCYSTEINE ACETYLGLUCOSAMINE ACETYLGLUTAMIC ACID ACETYL-L-LEUCINE ACETYLPHENYLALANINE ACETYLSEROTONIN ACETYLTRYPTOPHAN ACEXAMIC ACID ACIVICIN ACLACINOMYCIN A1 ACONITINE ACRIFLAVINIUM HYDROCHLORIDE ACRISORCIN ACTINONIN ACYCLOVIR ADENOSINE PHOSPHATE ADENOSINE ADRENALINE BITARTRATE AESCULIN AJMALINE AKLAVINE HYDROCHLORIDE ALANYL-dl-LEUCINE ALANYL-dl-PHENYLALANINE ALAPROCLATE ALBENDAZOLE ALBUTEROL ALEXIDINE HYDROCHLORIDE ALLANTOIN ALLOPURINOL ALMOTRIPTAN ALOIN ALPRENOLOL ALTRETAMINE ALVERINE CITRATE AMANTADINE HYDROCHLORIDE AMBROXOL HYDROCHLORIDE AMCINONIDE AMIKACIN SULFATE AMILORIDE HYDROCHLORIDE 3-AMINOBENZAMIDE gamma-AMINOBUTYRIC ACID AMINOCAPROIC ACID N- (2-AMINOETHYL)-4-CHLOROBENZAMIDE (RO-16-6491) AMINOGLUTETHIMIDE AMINOHIPPURIC ACID AMINOHYDROXYBUTYRIC ACID AMINOLEVULINIC ACID HYDROCHLORIDE AMINOPHENAZONE 3-AMINOPROPANESULPHONIC ACID AMINOPYRIDINE 9-AMINO-1,2,3,4-TETRAHYDROACRIDINE HYDROCHLORIDE AMINOTHIAZOLE AMIODARONE HYDROCHLORIDE AMIPRILOSE AMITRIPTYLINE HYDROCHLORIDE AMLODIPINE BESYLATE AMODIAQUINE DIHYDROCHLORIDE AMOXEPINE AMOXICILLIN AMPICILLIN SODIUM AMPROLIUM AMRINONE AMYGDALIN ANABASAMINE HYDROCHLORIDE ANABASINE HYDROCHLORIDE ANCITABINE HYDROCHLORIDE ANDROSTERONE SODIUM SULFATE ANIRACETAM ANISINDIONE ANISODAMINE ANISOMYCIN ANTAZOLINE PHOSPHATE ANTHRALIN ANTIMYCIN A (A1 shown) ANTIPYRINE APHYLLIC -

Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 BR 111 / 2017 The Minister responsible for health, in exercise of the power conferred by section 48A(1) of the Pharmacy and Poisons Act 1979, makes the following Order: Citation 1 This Order may be cited as the Pharmacy and Poisons (Third and Fourth Schedule Amendment) Order 2017. Repeals and replaces the Third and Fourth Schedule of the Pharmacy and Poisons Act 1979 2 The Third and Fourth Schedules to the Pharmacy and Poisons Act 1979 are repealed and replaced with— “THIRD SCHEDULE (Sections 25(6); 27(1))) DRUGS OBTAINABLE ONLY ON PRESCRIPTION EXCEPT WHERE SPECIFIED IN THE FOURTH SCHEDULE (PART I AND PART II) Note: The following annotations used in this Schedule have the following meanings: md (maximum dose) i.e. the maximum quantity of the substance contained in the amount of a medicinal product which is recommended to be taken or administered at any one time. 1 PHARMACY AND POISONS (THIRD AND FOURTH SCHEDULE AMENDMENT) ORDER 2017 mdd (maximum daily dose) i.e. the maximum quantity of the substance that is contained in the amount of a medicinal product which is recommended to be taken or administered in any period of 24 hours. mg milligram ms (maximum strength) i.e. either or, if so specified, both of the following: (a) the maximum quantity of the substance by weight or volume that is contained in the dosage unit of a medicinal product; or (b) the maximum percentage of the substance contained in a medicinal product calculated in terms of w/w, w/v, v/w, or v/v, as appropriate. -
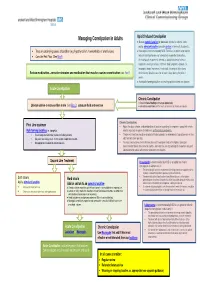
Managing Constipation in Adults
Managing Constipation in Adults Opio id Induced Constipation ● Use an osmotic laxative (or docusate which also softens stools and a stimulant laxative (consider dantron in terminally ill patients). • Treat any underlying causes of condition (e.g. hypothyroidism, haemorrhoids or anal fissures) ● Naloxegol is recommended by NICE TA345 as an option when opioid induced constipation has not adequately responded to laxatives. • Consider Red Flags (See Box A ) An inadequate response is defined as opioid-induced of at least moderate severity in at least 1of the 4 stool symptoms domain (i.e. incomplete bowel movement, hard stool, straining or false alarm) Review medication –consider stopping any medication that may be causing constipation (see Box B ) whilst taking1 laxative class for at least 4 days during the prior 2 weeks. ● Avoid bulk -forming l axatives for treating opioid induced constipation Acute Constipation Chronic Constipation ● Check for faecal loading and manage appropriately Lifestyle advise -to increase fibre in diet (see Box C ), adequate fluid and exercise ● Set realistic expectations for the results of treatment of chronic constipation. Chronic Constipation: First Line treatment • Adjust the dose, choice, and combination of laxative according to symptoms, speed with which Bulk forming laxatives i.e. ispaghula. relief is required, response to treatment, and individual preference. • Ensure adequate fluid intake (caution- frail elderly patients) • The dose of laxative should be gradually titrated upwards (or downwards) to produce one or two • May take several days to act- so not suitable if rapid relief required. soft, formed stools per day. • Not appropriate for opiod induced constipation. • If at least two laxatives (from different classes) have been tried at the highest tolerated recommended doses for at least 6 months, consider the use of prucalopride in women only and lubiprostone for adults with chronic idiopathic constipation. -

COMPASS Therapeutic Notes on the Management of Chronic Constipation in Primary Care
COMPASS Therapeutic Notes on the Management of Chronic Constipation in Primary Care In this issue: Glossary Page Chronic Constipation lasting longer than 3 months Introduction and background 1 constipation Drugs used in the management of Functional Chronic constipation without a known cause. Also known as primary 3 chronic constipation constipation constipation or idiopathic constipation Bulk-forming laxatives 4 Gastrocolic The occurrence of peristalsis following the entrance of food into the Osmotic laxatives 5 response empty stomach Stimulant laxatives 6 IBS Irritable Bowel Syndrome IBS-C Irritable Bowel Syndrome with Constipation Faecal softeners 7 Melanosis Dark brownish black pigmentation of the mucous membrane of the colon Peripheral opioid-receptor 7 coli due to the deposition of pigment in macrophages antagonists Myenteric Part of the enteric nervous system with an important role in regulating 5HT4 – receptor agonists 7 plexus gut motility Managing constipation in 8 NNT Number Needed to Treat pregnancy and breastfeeding OTC Over The Counter RCT Randomised Controlled Trial Secondary Constipation caused by a drug or medical condition. Secondary constipation constipation is also known as organic constipation SmPC Summary of Product Characteristics A twisting or looping of the bowel resulting in obstruction; can be life- Volvulus threatening Successful completion of the assessment questions at the end of this issue will provide you with 2 hours towards your CPD/CME requirements. Further copies of this and any other edition in the -

1: Gastro-Intestinal System
1 1: GASTRO-INTESTINAL SYSTEM Antacids .......................................................... 1 Stimulant laxatives ...................................46 Compound alginate products .................. 3 Docuate sodium .......................................49 Simeticone ................................................... 4 Lactulose ....................................................50 Antimuscarinics .......................................... 5 Macrogols (polyethylene glycols) ..........51 Glycopyrronium .......................................13 Magnesium salts ........................................53 Hyoscine butylbromide ...........................16 Rectal products for constipation ..........55 Hyoscine hydrobromide .........................19 Products for haemorrhoids .................56 Propantheline ............................................21 Pancreatin ...................................................58 Orphenadrine ...........................................23 Prokinetics ..................................................24 Quick Clinical Guides: H2-receptor antagonists .......................27 Death rattle (noisy rattling breathing) 12 Proton pump inhibitors ........................30 Opioid-induced constipation .................42 Loperamide ................................................35 Bowel management in paraplegia Laxatives ......................................................38 and tetraplegia .....................................44 Ispaghula (Psyllium husk) ........................45 ANTACIDS Indications: -

Pharmacy and Poisons Act 1979
Q UO N T FA R U T A F E BERMUDA PHARMACY AND POISONS ACT 1979 1979 : 26 TABLE OF CONTENTS PART I PRELIMINARY 1 Short title 2 Interpretation PART II THE PHARMACY COUNCIL 3 The Pharmacy Council 4 Membership of the Council 4A Functions of the Council 4B Protection from personal liability 4C Annual Report 5 Proceedings of the Council, etc PART III REGISTRATION OF PHARMACISTS 6 Offence to practise pharmacy if not registered 7 Registration as a pharmacist 7A Re-registration as non-practising member 7AA Period of validity of registration 8 Code of Conduct 9 Pharmacy Profession Complaints Committee 10 Investigation of complaint by Committee 10A Inquiry into complaint by Council 10B Inquiry by Council of its own initiative 11 Surrender of registration 12 Restoration of name to register 1 PHARMACY AND POISONS ACT 1979 13 Proof of registration 14 Appeals 14A Fees 14B Amendment of Seventh Schedule 15 Regulations for this part PART IV REGISTRATION OF PHARMACIES 16 Register of pharmacies 17 Registration of premises as registered pharmacies 18 Unfit premises: new applications 19 Unfit premises: registered pharmacies 20 Appeals 21 When certificates of unfitness take effect 22 Regulations for this Part PART V CONTROL OF PRESCRIPTIONS AND IMPORTATION 23 Prescriptions to be in a certain form 23A Validity of a prescription 24 Supply by registered pharmacist of equivalent medicines 25 Restrictions on the importation of medicines 26 Declaration relating to imported medicines [repealed] PART VI CONTROL OF DRUGS 27 Certain substances to be sold on prescription -

National Institute for Clinical Excellence
Appendix C NATIONAL INSTITUTE FOR HEALTH AND CARE EXCELLENCE Single Technology Appraisal (STA) Lubiprostone for treating opioid-induced constipation in people with chronic, non-cancer pain Matrix of consultees and commentators Consultees Commentators (no right to submit or appeal) Manufacturers/sponsors General Sucampo Pharma Europe Allied Health Professionals Federation (lubiprostone) Board of Community Health Councils in Wales Patient/carer groups British National Formulary Action on Pain Care Quality Commission Afiya Trust Commissioning Support Appraisals Black Health Agency Service Bladder and Bowel Foundation Department of Health, Social Services Equalities National Council and Public Safety for Northern Ireland Muslim Council of Britain Healthcare Improvement Scotland Muslim Health Network Medicines and Healthcare products Pain Concern Regulatory Agency Pain Relief Foundation National Association of Primary Care Pain UK National Pharmacy Association PromoCon NHS Alliance South Asian Health Foundation NHS Commercial Medicines Unit Specialised Healthcare Alliance NHS Confederation IBS Network Public Health Wales NHS Trust Scottish Medicines Consortium Professional groups Association of Coloproctology of Great Comparator manufacturers Britain and Ireland Abbott Laboratories UK (lactulose) Association for Continence Advice Actavis UK (glycerol suppositories), Association for Palliative Medicine Amdipharm (methylcellulose) British Geriatrics Society Bell Sons & Co (Druggists) Limited British Pain -

A Four-Country Comparison of Healthcare Systems, Implementation
Neurogastroenterology & Motility Neurogastroenterol Motil (2014) 26, 1368–1385 doi: 10.1111/nmo.12402 REVIEW ARTICLE A four-country comparison of healthcare systems, implementation of diagnostic criteria, and treatment availability for functional gastrointestinal disorders A report of the Rome Foundation Working Team on cross-cultural, multinational research M. SCHMULSON,* E. CORAZZIARI,† U. C. GHOSHAL,‡ S.-J. MYUNG,§ C. D. GERSON,¶ E. M. M. QUIGLEY,** K.-A. GWEE†† & A. D. SPERBER‡‡ *Laboratorio de Hıgado, Pancreas y Motilidad (HIPAM)-Department of Experimental Medicine, Faculty of Medicine-Universidad Nacional Autonoma de Mexico (UNAM). Hospital General de Mexico, Mexico City, Mexico †Gastroenterologia A, Department of Internal Medicine and Medical Specialties, University La Sapienza, Rome, Italy ‡Department of Gastroenterology, Sanjay Gandhi Postgraduate Institute of Medical Science, Lucknow, India §Department of Gastroenterology, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea ¶Division of Gastroenterology, Mount Sinai School of Medicine, New York, NY, USA **Division of Gastroenterology and Hepatology, Houston Methodist Hospital and Weill Cornell Medical College, Houston, TX, USA ††Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore ‡‡Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer-Sheva, Israel Key Messages • This report identified seven key issues related to healthcare provision that may impact how patients with FGIDs are investigated, diagnosed and managed. • Variations in healthcare provision around the world in patients with FGIDs have not been reviewed. • We compared four countries that are geographically and culturally diverse, and exhibit differences in the healthcare coverage provided to their population: Italy, South Korea, India and Mexico. • Since there is a paucity of publications relating to the issues covered in this report, some of the findings are based on the authors’ personal perspectives, press reports and other published sources. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Marrakesh Agreement Establishing the World Trade Organization
No. 31874 Multilateral Marrakesh Agreement establishing the World Trade Organ ization (with final act, annexes and protocol). Concluded at Marrakesh on 15 April 1994 Authentic texts: English, French and Spanish. Registered by the Director-General of the World Trade Organization, acting on behalf of the Parties, on 1 June 1995. Multilat ral Accord de Marrakech instituant l©Organisation mondiale du commerce (avec acte final, annexes et protocole). Conclu Marrakech le 15 avril 1994 Textes authentiques : anglais, français et espagnol. Enregistré par le Directeur général de l'Organisation mondiale du com merce, agissant au nom des Parties, le 1er juin 1995. Vol. 1867, 1-31874 4_________United Nations — Treaty Series • Nations Unies — Recueil des Traités 1995 Table of contents Table des matières Indice [Volume 1867] FINAL ACT EMBODYING THE RESULTS OF THE URUGUAY ROUND OF MULTILATERAL TRADE NEGOTIATIONS ACTE FINAL REPRENANT LES RESULTATS DES NEGOCIATIONS COMMERCIALES MULTILATERALES DU CYCLE D©URUGUAY ACTA FINAL EN QUE SE INCORPOR N LOS RESULTADOS DE LA RONDA URUGUAY DE NEGOCIACIONES COMERCIALES MULTILATERALES SIGNATURES - SIGNATURES - FIRMAS MINISTERIAL DECISIONS, DECLARATIONS AND UNDERSTANDING DECISIONS, DECLARATIONS ET MEMORANDUM D©ACCORD MINISTERIELS DECISIONES, DECLARACIONES Y ENTEND MIENTO MINISTERIALES MARRAKESH AGREEMENT ESTABLISHING THE WORLD TRADE ORGANIZATION ACCORD DE MARRAKECH INSTITUANT L©ORGANISATION MONDIALE DU COMMERCE ACUERDO DE MARRAKECH POR EL QUE SE ESTABLECE LA ORGANIZACI N MUND1AL DEL COMERCIO ANNEX 1 ANNEXE 1 ANEXO 1 ANNEX