A Revised Generic Classification of Vittarioid Ferns (Pteridaceae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

^ a Critical Examination of the Vittarieae with a View To
/ P u n -t/p '& . J9. ^ _ ^ 2 (tO. A CRITICAL EXAMINATION OF THE VITTARIEAE WITH A VIEW TO THEIR SYSTEMATIC COMPARISON. 1. Introduction. 2. Vittaria. 3. Monogramma, 4. Antrophyum. ProQuest Number: 13916247 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 13916247 Published by ProQuest LLC(2019). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 INTRODUCTION. The Vittarieae, as described by Christensen (1), comprises five genera, viz. Vjfrfrftrja, Monogramma,. Antronhvum. Hecistopterist and Anetium. all of whicij are epiphytic forms growing in the damp forests of the Old and New;World Tropics. All of them possess creeping rhizomes on which the frortds are arranged more or less definitely in two rows on the dorsal surface. The fronds are simple in outline with the exception of those of Heoistonteris which are dichotomously branched. The venation of the fronds is reticulate except in Heoistonteris where there is an open, dichotomous system of veins^An interesting feature, which has proved to be valuable as a diagnostic character, is the presence of "spicule cells" in the epidermis. -

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -

Pteris Carsei
Pteris carsei COMMON NAME Coastal brake, netted brake SYNONYMS Pteris comans G.Forst. (misapplied name) FAMILY Pteridaceae AUTHORITY Pteris carsei Braggins et Brownsey FLORA CATEGORY Vascular – Native ENDEMIC TAXON Yes ENDEMIC GENUS Pteris carsei Motuoruhi, Coromandel. No Photographer: John Smith-Dodsworth ENDEMIC FAMILY No STRUCTURAL CLASS Ferns NVS CODE PTECAR CHROMOSOME NUMBER 2n = 58, 60 Pteris carsei Motuoruhi, Coromandel. Photographer: John Smith-Dodsworth CURRENT CONSERVATION STATUS 2012 | Not Threatened PREVIOUS CONSERVATION STATUSES 2009 | Not Threatened 2004 | Not Threatened DISTRIBUTION Endemic. New Zealand: Kermadec Islands (Raoul, the Meyers Islands and Macauley Island), Three Kings and North Island from North Cape to Bay of Plenty in the east and Awhitu Peninsula in the west with an outlying population near Mokau. HABITAT Coastal in forest especially on the sides of gullies, on banks and in valley heads. A very common offshore island fern FEATURES Terrestrial ferns. Rhizomes short, erect, scaly. Stipes 0.25-0.6 m long, pale brown, glabrous or scaly at very base. Laminae 0.2-1.8 × 0.15-0.9 m, dark green to yellow-green, 2-3-pinnate at base, ovate, coriaceous, veins reticulate. Pinnae not overlapping; most lower secondary pinnae adnate. Ultimate segments 10-55 × 5-10 mm, oblong, apices tapering or bluntly pointed, margins toothed. Sori continuous along pinna margins on a marginal vein, protected by a membranous inrolled pinna margins. SIMILAR TAXA Pteris carsei is easily distinguished from all other New Zealand Pteris by the coriaceous (leathery) fronds, reticulate venation, overlapping pinnae and large ultimate segments. The only other Pteris with reticulate venation are P. -

Metabolic Adaptations to Arsenic-Induced Oxidative Stress in Pterisvittata L and Pterisensiformis L
Plant Science 170 (2006) 274–282 www.elsevier.com/locate/plantsci Metabolic adaptations to arsenic-induced oxidative stress in Pteris vittata L and Pteris ensiformis L Nandita Singh a, Lena Q. Ma b,*, Mrittunjai Srivastava b, Bala Rathinasabapathi c a National Botanical Research Institute, Rana Pratap Marg, Lucknow 226001, India b Soil and Water Science Department, University of Florida, Box 110290, Gainesville, FL 32611-0290, USA c Horticultural Sciences Department, University of Florida, Gainesville, FL 32611, USA Received 14 July 2005; received in revised form 12 August 2005; accepted 19 August 2005 Available online 8 September 2005 Abstract This study examined the metabolic adaptations of Pteris vittata L, an arsenic hyperaccumulator, under arsenic stress as compared to Pteris ensiformis, a non-arsenic hyperaccumulator. Both plants were grown hydroponically in 20% Hoagland medium in controlled conditions and were treated with 0, 133 or 267 mM arsenic as sodium arsenate for 1, 5 or 10 d. The fern fronds were analysed for differences in oxidative stress and antioxidant capacities after arsenic exposure. Upon exposure to 133 mM arsenic, concentrations of chlorophyll, protein and carotenoids increased in P. vittata whereas they decreased in P. ensiformis. The H2O2 and TBARs concentrations were greater in P. ensiformis than P. vittata in all treatments, indicating greater production of reactive oxygen species (ROS) by P. ensiformis. The levels of ascorbate and glutathione, and their reduced/oxidized ratios in the fronds of P. vittata of the control was much greater than P. ensiformis indicating that P. vittata has an inherently greater antioxidant potential than P. ensiformis. The lower levels of antioxidant compounds (ascorbate, carotenoids and glutathione) in P. -

Genetic Differentiation and Polyploid Formation Within the Cryptogramma Crispa Complex (Polypodiales: Pteridaceae)
Turkish Journal of Botany Turk J Bot (2016) 40: 231-240 http://journals.tubitak.gov.tr/botany/ © TÜBİTAK Research Article doi:10.3906/bot-1501-54 Genetic differentiation and polyploid formation within the Cryptogramma crispa complex (Polypodiales: Pteridaceae) Jordan METZGAR*, Mackenzie STAMEY, Stefanie ICKERT-BOND Herbarium (ALA), University of Alaska Museum of the North and Department of Biology and Wildlife, University of Alaska Fairbanks, Fairbanks, AK, USA Received: 28.01.2015 Accepted/Published Online: 14.07.2015 Final Version: 08.04.2016 Abstract: The tetraploid fern Cryptogramma crispa (L.) R.Br. ex Hook. is distributed across alpine and high latitude regions of Europe and western Asia and is sympatric with the recently described octoploid C. bithynica S.Jess., L.Lehm. & Bujnoch in north-central Turkey. Our analysis of a 6-region plastid DNA sequence dataset comprising 39 accessions of Cryptogramma R.Br., including 14 accessions of C. crispa and one accession of C. bithynica, revealed a deep genetic division between the accessions of C. crispa from western, northern, and central Europe and the accessions of C. crispa and C. bithynica from Turkey and the Caucasus Mountains. This legacy likely results from Pleistocene climate fluctuations and appears to represent incipient speciation between the eastern and western clades. These plastid DNA sequence data also demonstrate that the western clade of C. crispa, specifically the western Asian clade, is the maternal progenitor of C. bithynica. Our analysis of DNA sequence data from the biparentally inherited nuclear locus gapCp supports an autopolyploid origin of C. bithynica, with C. crispa as the sole progenitor. Key words: Cryptogramma, ferns, autopolyploidy, phylogeography, glacial refugium 1. -

Species Relationships and Farina Evolution in the Cheilanthoid Fern
Systematic Botany (2011), 36(3): pp. 554–564 © Copyright 2011 by the American Society of Plant Taxonomists DOI 10.1600/036364411X583547 Species Relationships and Farina Evolution in the Cheilanthoid Fern Genus Argyrochosma (Pteridaceae) Erin M. Sigel , 1 , 3 Michael D. Windham , 1 Layne Huiet , 1 George Yatskievych , 2 and Kathleen M. Pryer 1 1 Department of Biology, Duke University, Durham, North Carolina 27708 U. S. A. 2 Missouri Botanical Garden, P.O. Box 299, St. Louis, Missouri 63166 U. S. A. 3 Author for correspondence ( [email protected] ) Communicating Editor: Lynn Bohs Abstract— Convergent evolution driven by adaptation to arid habitats has made it difficult to identify monophyletic taxa in the cheilanthoid ferns. Dependence on distinctive, but potentially homoplastic characters, to define major clades has resulted in a taxonomic conundrum: all of the largest cheilanthoid genera have been shown to be polyphyletic. Here we reconstruct the first comprehensive phylogeny of the strictly New World cheilanthoid genus Argyrochosma . We use our reconstruction to examine the evolution of farina (powdery leaf deposits), which has played a prominent role in the circumscription of cheilanthoid genera. Our data indicate that Argyrochosma comprises two major monophyletic groups: one exclusively non-farinose and the other primarily farinose. Within the latter group, there has been at least one evolutionary reversal (loss) of farina and the development of major chemical variants that characterize specific clades. Our phylogenetic hypothesis, in combination with spore data and chromosome counts, also provides a critical context for addressing the prevalence of polyploidy and apomixis within the genus. Evidence from these datasets provides testable hypotheses regarding reticulate evolution and suggests the presence of several previ- ously undetected taxa of Argyrochosma. -

Insights on Reticulate Evolution in Ferns, with Special Emphasis on the Genus Ceratopteris
Utah State University DigitalCommons@USU All Graduate Theses and Dissertations Graduate Studies 8-2021 Insights on Reticulate Evolution in Ferns, with Special Emphasis on the Genus Ceratopteris Sylvia P. Kinosian Utah State University Follow this and additional works at: https://digitalcommons.usu.edu/etd Part of the Ecology and Evolutionary Biology Commons Recommended Citation Kinosian, Sylvia P., "Insights on Reticulate Evolution in Ferns, with Special Emphasis on the Genus Ceratopteris" (2021). All Graduate Theses and Dissertations. 8159. https://digitalcommons.usu.edu/etd/8159 This Dissertation is brought to you for free and open access by the Graduate Studies at DigitalCommons@USU. It has been accepted for inclusion in All Graduate Theses and Dissertations by an authorized administrator of DigitalCommons@USU. For more information, please contact [email protected]. INSIGHTS ON RETICULATE EVOLUTION IN FERNS, WITH SPECIAL EMPHASIS ON THE GENUS CERATOPTERIS by Sylvia P. Kinosian A dissertation submitted in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in Ecology Approved: Zachariah Gompert, Ph.D. Paul G. Wolf, Ph.D. Major Professor Committee Member William D. Pearse, Ph.D. Karen Mock, Ph.D Committee Member Committee Member Karen Kaphiem, Ph.D Michael Sundue, Ph.D. Committee Member Committee Member D. Richard Cutler, Ph.D. Interim Vice Provost of Graduate Studies UTAH STATE UNIVERSITY Logan, Utah 2021 ii Copyright © Sylvia P. Kinosian 2021 All Rights Reserved iii ABSTRACT Insights on reticulate evolution in ferns, with special emphasis on the genus Ceratopteris by Sylvia P. Kinosian, Doctor of Philosophy Utah State University, 2021 Major Professor: Zachariah Gompert, Ph.D. -

National List of Vascular Plant Species That Occur in Wetlands 1996
National List of Vascular Plant Species that Occur in Wetlands: 1996 National Summary Indicator by Region and Subregion Scientific Name/ North North Central South Inter- National Subregion Northeast Southeast Central Plains Plains Plains Southwest mountain Northwest California Alaska Caribbean Hawaii Indicator Range Abies amabilis (Dougl. ex Loud.) Dougl. ex Forbes FACU FACU UPL UPL,FACU Abies balsamea (L.) P. Mill. FAC FACW FAC,FACW Abies concolor (Gord. & Glend.) Lindl. ex Hildebr. NI NI NI NI NI UPL UPL Abies fraseri (Pursh) Poir. FACU FACU FACU Abies grandis (Dougl. ex D. Don) Lindl. FACU-* NI FACU-* Abies lasiocarpa (Hook.) Nutt. NI NI FACU+ FACU- FACU FAC UPL UPL,FAC Abies magnifica A. Murr. NI UPL NI FACU UPL,FACU Abildgaardia ovata (Burm. f.) Kral FACW+ FAC+ FAC+,FACW+ Abutilon theophrasti Medik. UPL FACU- FACU- UPL UPL UPL UPL UPL NI NI UPL,FACU- Acacia choriophylla Benth. FAC* FAC* Acacia farnesiana (L.) Willd. FACU NI NI* NI NI FACU Acacia greggii Gray UPL UPL FACU FACU UPL,FACU Acacia macracantha Humb. & Bonpl. ex Willd. NI FAC FAC Acacia minuta ssp. minuta (M.E. Jones) Beauchamp FACU FACU Acaena exigua Gray OBL OBL Acalypha bisetosa Bertol. ex Spreng. FACW FACW Acalypha virginica L. FACU- FACU- FAC- FACU- FACU- FACU* FACU-,FAC- Acalypha virginica var. rhomboidea (Raf.) Cooperrider FACU- FAC- FACU FACU- FACU- FACU* FACU-,FAC- Acanthocereus tetragonus (L.) Humm. FAC* NI NI FAC* Acanthomintha ilicifolia (Gray) Gray FAC* FAC* Acanthus ebracteatus Vahl OBL OBL Acer circinatum Pursh FAC- FAC NI FAC-,FAC Acer glabrum Torr. FAC FAC FAC FACU FACU* FAC FACU FACU*,FAC Acer grandidentatum Nutt. -

Morphological and Anatomical Adaptations to Dry, Shady Environments in Adiantum Reniforme Var
Morphological and anatomical adaptations to dry, shady environments in Adiantum reniforme var. sinense (Pteridaceae) Di Wu1, Linbao Li1, Xiaobo Ma1, Guiyun Huang1 and Chaodong Yang2 1 Rare Plants Research Institute of Yangtze River, Three Gorges Corporation, Yichang, China 2 Engineering Research Center of Ecology and Agriculture Use of Wetland, Ministry of Education, Yangtze University, Jingzhou, China ABSTRACT The natural distribution of the rare perennial fern Adiantum reniforme var. sinense (Pteridaceae), which is endemic to shady cliff environments, is limited to small areas of Wanzhou County, Chongqing, China. In this study, we used brightfield and epifluorescence microscopy to investigate the anatomical structures and histochemical features that may allow this species to thrive in shady, dry cliff environments. The A. reniforme var. sinense sporophyte had a primary structure and a dictyostele. The plants of this species had an endodermis, sclerenchyma layers and hypodermal sterome, reflecting an adaption to dry cliff environments. Blades had a thin cuticle and isolateral mesophyll, suggesting a tolerance of shady environments. These characteristics are similar to many sciophyte ferns such as Lygodium japonicum and Pteris multifida. Thus, the morphological and anatomical characteristics of A. reniforme var. sinense identified in this study are consistent with adaptations to shady, dry cliff environments. Subjects Conservation Biology, Plant Science Keywords Endodermis, Dictyostele, Sclerenchyma layer, Suberin lamellae, Thin cuticle Submitted 14 April 2020 Accepted 24 August 2020 INTRODUCTION Published 30 September 2020 Adiantum reniforme var. sinense (Pteridaceae, subfamily Vittarioideae) is a rare Corresponding authors Guiyun Huang, cliff-dwelling perennial pteridophyte, with a natural distribution limited to small areas of [email protected] Wanzhou County, Chongqing, China. -

11-122. 2000 11
FERN GAZ. 16(1, 2)11-122. 2000 11 CHECKLIST OF THE PTERIDOPHYTES OF TRINIDAD & TOBAGO Y. S. BAKSH-COMEAU The National Herbarium of Trinidad and Tobago. Department of Life Sciences, The University of the West Indies, St. Augustine, Trinidad, West Indies Key words: checklist, Trinidad and Tobago pteridophytes, types, habitat, distribution. ABSTRACT Three hundred and two species and eight varieties or subspecies in 27 families and 77 genera of ferns and fern allies are listed. Four new combinations and states are made, and one synonym lectotypified. A serious attempt has been made to establish types; selections of specimens studied are cited. INTRODUCTION Recent studies of ferns in Trinidad and Tobago (Baksh-Comeau, 1996, 1999) have combined a review of the pteridophyte collection at The National Herbarium of Trinidad & Tobago with field surveys undertaken to assess the community status of these plants on both islands. This checklist has been developed as an integral part of those studies, but it is also an essential prerequisite to ongoing research covering a reclassification of the vegetation of the islands and to the preparation of a comprehensive vascular plant flora. The herbarium count and field survey revealed 251 species confirmed by voucher specimens housed in Trinidad. Additional species have been attributed to Trinidad or Tobago in early publications for Trinidad and in Floras and monographs for neighbouring areas. The number of species now believed to be indigenous in these islands is 282. Cultivated species that have escaped, and introductions which have become naturalized number 20. Early reports include Grisebach (1859-64) who listed 106 species; Eaton (1878) approximately 78 of the 150 or so species eventually collected by August Fendler; Jenman (1887) had about 184 species; Anon (1889) listed 206 binomials including a few introduced taxa; Jenman (1898-1909), in an incomplete coverage of the fern flora, described 140 taxa of which 10 were new species; Hart (1908), including some cultivated plants, listed 283 binomials of pteridophytes. -
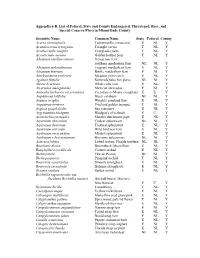
Conservation Appendix 6-B Listed Flora
Appendix 6-B. List of Federal, State and County Endangered, Threatened, Rare, and Special Concern Flora in Miami-Dade County Scientific Name Common Name State Federal County Acacia choriophylla Tamarindillo; cinnecord E NL Y Acanthocereus tetragenus Triangle cactus T NL Y Acoelorraphe wrightii Everglades palm T NL Y Acrostichum aureum Golden leather fern T NL Y Adiantum capillus-veneris Venus hair fern; southern maidenhair fern NL NL Y Adiantum melanoleucum Fragrant maidenhair fern E NL Y Adiantum tenerum Brittle maidenhair fern E NL Y Aeschynomene pratensis Meadow joint-vetch E NL Y Agalinis filifolia Seminole false fox glove NL NL Y Aletris bracteata White colic root E NL Y Alvaradoa amorphoides Mexican alvaradoa E NL Y Amorpha herbacea var.crenulata Crenulate (=Miami) leadplant E E Y Amphitecna latifolia Black calabash NL NL Y Anemia wrightii Wright's pineland fern E NL Y Angadenia berteroi Pineland golden trumpet T NL Y Argusia gnaphalodes Sea rosemary E NL Y Argythamnia blodgettii Blodgett's silverbush E C Y Aristolochia pentandra Marsh's dutchmans pipe E NL Y Asplenium abscissum Cutleaf spleenwort NL NL Y Asplenium dentatum Toothed spleenwort E NL Y Asplenium serratum Wild bird nest fern E NL Y Asplenium verecundum Modest spleenwort E NL Y Asplenium x biscaynianum Biscayne spleenwort NL NL Y Asteraea lobata Lobed croton; Florida treefern NL NL Y Baccharis dioica Broombush falsewillow E NL Y Basiphyllaea corallicola Carter's orchid E NL Y Bletia patula Flor de Pesmo NL NL Y Bletia purpurea Pinepink orchid T NL Y Bourreria cassinifolia Smooth strongback E NL Y Bourreria succulenta Bahama strongback E NL Y Brassia caudata Spider orchid E NL Y Brickellia eupatorioides var. -

Epiphytes and the National Wetland Plant List
Lichvar, R.W. and W. Fertig. 2011. Epiphytes and the National Wetland Plant List. Phytoneuron 2011-16: 1–31. EPIPHYTES AND THE NATIONAL WETLAND PLANT LIST ROBERT W. LICHVAR U.S. Army Engineer Research and Development Center Cold Regions Research and Engineering Laboratory 72 Lyme Road Hanover, NH 03755-1290 WALTER FERTIG Moenave Botanical Consulting 1117 West Grand Canyon Drive Kanab, UT 84741 ABSTRACT The National Wetland Plant List (NWPL) is a list of species that occur in wetlands in the United States. It is a product of a collaborative effort of four Federal agencies: the U.S. Army Corps of Engineers, the U.S. Environmental Protection Agency, the U.S. Fish and Wildlife Service, and the Natural Resources Conservation Service. The NWPL has many uses, but it is specifically designed for use in wetland delineation for establishing the extent of Federal jurisdictional of wetland boundaries. To be listed in the NWPL, a plant must be rooted in soil, so there is a direct relationship between a plant’s occurrence and its preference for hydric soils. This relationship, coupled with the plant’s frequency of occurrence in wetlands, is used to place it in one of five categories representing the probability that the plant occurs in a wetland. Many species are considered to be epiphytes, but they represent various life forms, ranging from purely epiphytic to frequently occurring on the ground. Based on a literature review of 192 species across the United States and its territories, we determined which species fell into four categories of epiphytic life forms or are terrestrial and should not be considered epiphytes.