Valence Bond Theory: Hybridization
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Shapes and Structures of Organic Molecules HYBRIDISATION
Department of Chemistry Anugrah Memorial College, Gaya Class- B.Sc. 1 (Hons) Subject- Organic Chemistry Paper- 1C Unit- Shapes and Structures of Organic molecules Teacher – Dr. Nidhi Tripathi Assistant Professor Shapes and Structures of Organic molecules HYBRIDISATION The formation of bonds is no less than the act of courtship. Atoms come closer, attract to each other and gradually lose a little part of themselves to the other atoms. In chemistry, the study of bonding, that is, Hybridization is of prime importance. What happens to the atoms during bonding? What happens to the atomic orbitals? The answer lies in the concept of Hybridisation. Let us see! Introducing Hybridisation All elements around us, behave in strange yet surprising ways. The electronic configuration of these elements, along with their properties, is a unique concept to study and observe. Owing to the uniqueness of such properties and uses of an element, we are able to derive many practical applications of such elements. When it comes to the elements around us, we can observe a variety of physical properties that these elements display. The study of hybridization and how it allows the combination of various molecules in an interesting way is a very important study in science. Understanding the properties of hybridisation lets us dive into the realms of science in a way that is hard to grasp in one go but excellent to study once we get to know more about it. Let us get to know more about the process of hybridization, which will help us understand the properties of different elements. What is Hybridization? Scientist Pauling introduced the revolutionary concept of hybridization in the year 1931. -

VSEPR Theory
VSEPR Theory The valence-shell electron-pair repulsion (VSEPR) model is often used in chemistry to predict the three dimensional arrangement, or the geometry, of molecules. This model predicts the shape of a molecule by taking into account the repulsion between electron pairs. This handout will discuss how to use the VSEPR model to predict electron and molecular geometry. Here are some definitions for terms that will be used throughout this handout: Electron Domain – The region in which electrons are most likely to be found (bonding and nonbonding). A lone pair, single, double, or triple bond represents one region of an electron domain. H2O has four domains: 2 single bonds and 2 nonbonding lone pairs. Electron Domain may also be referred to as the steric number. Nonbonding Pairs Bonding Pairs Electron domain geometry - The arrangement of electron domains surrounding the central atom of a molecule or ion. Molecular geometry - The arrangement of the atoms in a molecule (The nonbonding domains are not included in the description). Bond angles (BA) - The angle between two adjacent bonds in the same atom. The bond angles are affected by all electron domains, but they only describe the angle between bonding electrons. Lewis structure - A 2-dimensional drawing that shows the bonding of a molecule’s atoms as well as lone pairs of electrons that may exist in the molecule. Provided by VSEPR Theory The Academic Center for Excellence 1 April 2019 Octet Rule – Atoms will gain, lose, or share electrons to have a full outer shell consisting of 8 electrons. When drawing Lewis structures or molecules, each atom should have an octet. -

Valence Bond Theory
UMass Boston, Chem 103, Spring 2006 CHEM 103 Molecular Geometry and Valence Bond Theory Lecture Notes May 2, 2006 Prof. Sevian Announcements z The final exam is scheduled for Monday, May 15, 8:00- 11:00am It will NOT be in our regularly scheduled lecture hall (S- 1-006). The final exam location has been changed to Snowden Auditorium (W-1-088). © 2006 H. Sevian 1 UMass Boston, Chem 103, Spring 2006 More announcements Information you need for registering for the second semester of general chemistry z If you will take it in the summer: z Look for chem 104 in the summer schedule (includes lecture and lab) z If you will take it in the fall: z Look for chem 116 (lecture) and chem 118 (lab). These courses are co-requisites. z If you plan to re-take chem 103, in the summer it will be listed as chem 103 (lecture + lab). In the fall it will be listed as chem 115 (lecture) + chem 117 (lab), which are co-requisites. z Note: you are only eligible for a lab exemption if you previously passed the course. Agenda z Results of Exam 3 z Molecular geometries observed z How Lewis structure theory predicts them z Valence shell electron pair repulsion (VSEPR) theory z Valence bond theory z Bonds are formed by overlap of atomic orbitals z Before atoms bond, their atomic orbitals can hybridize to prepare for bonding z Molecular geometry arises from hybridization of atomic orbitals z σ and π bonding orbitals © 2006 H. Sevian 2 UMass Boston, Chem 103, Spring 2006 Molecular Geometries Observed Tetrahedral See-saw Square planar Square pyramid Lewis Structure Theory -

Transcription 12.02.15
Lecture 12A • 02/15/12 We’re going to continue our discussion of conjugation. If we go back and talk about electrons and how they act like waves, [there’s] something known as the particle in a box. You’ve got some kind of box where imagine that the walls are infinitely tall. It’s a one-dimensional system, where the electron, all it can do, is go left and right between the two walls of the box. The electron’s a wave; it can’t exist outside the box, so whatever function, whatever wave we use to describe the electron, has to have a value of zero at one end of the box and zero at the other end of the box. Graphically, what do the solutions look like? We have something like this, where we’re talking about energy potential; it’s infinite at either end of the box, and zero in between. We have an electron that’s bouncing around inside of it. An electron’s a wave, and there’s function that describes that wave. The only way it can fit in this box and physically make sense is if the wave starts and stops and the ends of the box. It turns out that – long, long, long story short – one of the solutions for the Schrödinger equation is just a sine function – actually, it’s part of an exponential version of a sine function. It’s something, at least, we can draw a pretty picture of. That’s why you’ll start with this problem in a discussion of quantum mechanics, because it is solvable, it is only on in dimension, and it’s more humanly possible to discuss. -

Aromaticity Sem- Ii
AROMATICITY SEM- II In 1931, German chemist and physicist Sir Erich Hückel proposed a theory to help determine if a planar ring molecule would have aromatic properties .This is a very popular and useful rule to identify aromaticity in monocyclic conjugated compound. According to which a planar monocyclic conjugated system having ( 4n +2) delocalised (where, n = 0, 1, 2, .....) electrons are known as aromatic compound . For example: Benzene, Naphthalene, Furan, Pyrrole etc. Criteria for Aromaticity 1) The molecule is cyclic (a ring of atoms) 2) The molecule is planar (all atoms in the molecule lie in the same plane) 3) The molecule is fully conjugated (p orbitals at every atom in the ring) 4) The molecule has 4n+2 π electrons (n=0 or any positive integer Why 4n+2π Electrons? According to Hückel's Molecular Orbital Theory, a compound is particularly stable if all of its bonding molecular orbitals are filled with paired electrons. - This is true of aromatic compounds, meaning they are quite stable. - With aromatic compounds, 2 electrons fill the lowest energy molecular orbital, and 4 electrons fill each subsequent energy level (the number of subsequent energy levels is denoted by n), leaving all bonding orbitals filled and no anti-bonding orbitals occupied. This gives a total of 4n+2π electrons. - As for example: Benzene has 6π electrons. Its first 2π electrons fill the lowest energy orbital, and it has 4π electrons remaining. These 4 fill in the orbitals of the succeeding energy level. The criteria for Antiaromaticity are as follows: 1) The molecule must be cyclic and completely conjugated 2) The molecule must be planar. -

Structures and Properties of Substances
Structures and Properties of Substances Introducing Valence-Shell Electron- Pair Repulsion (VSEPR) Theory The VSEPR theory In 1957, the chemist Ronald Gillespie and Ronald Nyholm, developed a model for predicting the shape of molecules. This model is usually abbreviated to VSEPR (pronounced “vesper”) theory: Valence Shell Electron Pair Repulsion The fundamental principle of the VSEPR theory is that the bonding pairs (BP) and lone pairs (LP) of electrons in the valence level of an atom repel one another. Thus, the orbital for each electron pair is positioned as far from the other orbitals as possible in order to achieve the lowest possible unstable structure. The effect of this positioning minimizes the forces of repulsion between electron pairs. A The VSEPR theory The repulsion is greatest between lone pairs (LP-LP). Bonding pairs (BP) are more localized between the atomic nuclei, so they spread out less than lone pairs. Therefore, the BP-BP repulsions are smaller than the LP-LP repulsions. The repulsion between a bond pair and a lone-pair (BP-LP) is intermediate between the other two. In other words, in terms of decreasing repulsion: LP-LP > LP-BP > BP-BP The tetrahedral shape around a single-bonded carbon atom (e.g. in CH4), the planar shape around a carbon atom with two double bond (e.g. in CO2), and the bent shape around an oxygen atom in H2O result from repulsions between lone pairs and/or bonding pairs of electrons. The VSEPR theory The repulsion is greatest between lone pairs (LP-LP). Bonding pairs (BP) are more localized between the atomic nuclei, so they spread out less than lone pairs. -

Lewis Structure Handout
Lewis Structure Handout for Chemistry Students An electron dot diagram, also known as a Lewis Structure, is a representation of valence electrons in a single atom, and can be further utilized to depict the bonds that form based on the available electrons for covalent bonding between multiple atoms. -Each atom has a characteristic dot diagram based on its position in the periodic table and this reflects its potential for fulfillment of the octet rule. As a general rule, the noble gases have a filled octet (column VIII with eight valence electrons) and each preceding column has successively one fewer. • For example, Carbon is in column IV and has four valence electrons. It can therefore be represented as: • Each of these "lone" electrons can form a bond by sharing the orbital with another unbonded electron. Hydrogen, being in column I, has a single valence electron, and will therefore satisfy carbon's octet thusly: • When a compound has bonded electrons (as with CH4) it is most accurate to diagram it with lines representing each of the single bonds. • Using carbon again for a slightly more complicated example, it can also form double bonds. This is the case when carbon bonds to two oxygen atoms, resulting in CO2. The oxygen atoms (with six valence electrons in column VI) are each represented as: • Because carbon is the least electronegative of the atoms involved, it is placed centrally, and its valence electrons will migrate toward the more electronegative oxygens to form two double bonds and a linear structure. This can be written as: where oxygen's remaining lone pairs are still shown. -

Introduction to Molecular Orbital Theory
Chapter 2: Molecular Structure and Bonding Bonding Theories 1. VSEPR Theory 2. Valence Bond theory (with hybridization) 3. Molecular Orbital Theory ( with molecualr orbitals) To date, we have looked at three different theories of molecular boning. They are the VSEPR Theory (with Lewis Dot Structures), the Valence Bond theory (with hybridization) and Molecular Orbital Theory. A good theory should predict physical and chemical properties of the molecule such as shape, bond energy, bond length, and bond angles.Because arguments based on atomic orbitals focus on the bonds formed between valence electrons on an atom, they are often said to involve a valence-bond theory. The valence-bond model can't adequately explain the fact that some molecules contains two equivalent bonds with a bond order between that of a single bond and a double bond. The best it can do is suggest that these molecules are mixtures, or hybrids, of the two Lewis structures that can be written for these molecules. This problem, and many others, can be overcome by using a more sophisticated model of bonding based on molecular orbitals. Molecular orbital theory is more powerful than valence-bond theory because the orbitals reflect the geometry of the molecule to which they are applied. But this power carries a significant cost in terms of the ease with which the model can be visualized. One model does not describe all the properties of molecular bonds. Each model desribes a set of properties better than the others. The final test for any theory is experimental data. Introduction to Molecular Orbital Theory The Molecular Orbital Theory does a good job of predicting elctronic spectra and paramagnetism, when VSEPR and the V-B Theories don't. -

8.3 Bonding Theories >
8.3 Bonding Theories > Chapter 8 Covalent Bonding 8.1 Molecular Compounds 8.2 The Nature of Covalent Bonding 8.3 Bonding Theories 8.4 Polar Bonds and Molecules 1 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals Molecular Orbitals How are atomic and molecular orbitals related? 2 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals • The model you have been using for covalent bonding assumes the orbitals are those of the individual atoms. • There is a quantum mechanical model of bonding, however, that describes the electrons in molecules using orbitals that exist only for groupings of atoms. 3 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals • When two atoms combine, this model assumes that their atomic orbitals overlap to produce molecular orbitals, or orbitals that apply to the entire molecule. 4 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals Just as an atomic orbital belongs to a particular atom, a molecular orbital belongs to a molecule as a whole. • A molecular orbital that can be occupied by two electrons of a covalent bond is called a bonding orbital. 5 Copyright © Pearson Education, Inc., or its affiliates. All Rights Reserved. 8.3 Bonding Theories > Molecular Orbitals Sigma Bonds When two atomic orbitals combine to form a molecular orbital that is symmetrical around the axis connecting two atomic nuclei, a sigma bond is formed. • Its symbol is the Greek letter sigma (σ). -
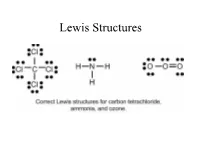
Lewis Structures
Lewis Structures Valence electrons for Elements Recall that the valence electrons for the elements can be determined based on the elements position on the periodic table. Lewis Dot Symbol Valence electrons and number of bonds Number of bonds elements prefers depending on the number of valence electrons. In general - F a m i l y → # C o v a l e n t B o n d s* H a l o g e n s X F , B r , C l , I → 1 bond often C a l c o g e n s O 2 bond often O , S → N i t r o g e n N 3 bond often N , P → C a r b o n C → 4 bond always C , S i The above chart is a guide on the number of bonds formed by these atoms. Lewis Structure, Octet Rule Guidelines When compounds are formed they tend to follow the Octet Rule. Octet Rule: Atoms will share electrons (e-) until it is surrounded by eight valence electrons. 4 unpaired 3unpaired 2unpaired 1unpaired up = unpaired e- 4 bonds 3 bonds 2 bonds 1 bond O=C=O N≡ N O = O F - F Atomic Connectivity The atomic arrangement for a molecule is usually given. CH2ClF HNO3 CH3COOH H2SO4 Cl N H O O O O H C F O H C C H O S O H H H H O H O In general when there is a single central atom in the 3- molecule, CH2ClF, SeCl2, O3 (CO2, NH3, PO4 ), the central atom is the first atom in the chemical formula. -

Draw Three Resonance Structures for the Chlorate Ion, Clo3
University Chemistry Quiz 3 2014/11/6 + 1. (10%) Explain why the bond order of N2 is greater than that of N2 , but the bond + order of O2 is less than that of O2 . Sol. + In forming the N2 from N2, an electron is removed from the sigma bonding molecular orbital. Consequently, the bond order decreases to 2.5 from 3.0. In + forming the O2 ion from O2, an electron is removed from the pi antibonding molecular orbital. Consequently, the bond order increases to 2.5 from 2.0. - 2. (10%) Draw three resonance structures for the chlorate ion, ClO3 . Show formal charges. Sol. Strategy: We follow the procedure for drawing Lewis structures outlined in Section 3.4 of the text. After we complete the Lewis structure, we draw the resonance structures. Solution: Following the procedure in Section 3.4 of the text, we come up with − the following Lewis structure for ClO3 . O − + − O Cl O We can draw two more equivalent Lewis structures with the double bond between Cl and a different oxygen atom. The resonance structures with formal charges are as follows: − − O O O + − − + − − + O Cl O O Cl O O Cl O 3. (5%) Use the molecular orbital energy-level diagram for O2 to show that the following Lewis structure corresponds to an excited state: Sol. The Lewis structure shows 4 pairs of electrons on the two oxygen atoms. From Table 3.4 of the text, we see that these 8 valence electrons are placed in the σ p p p p 2p , 2p , 2p , 2p , and 2p orbitals. -

Molecular Orbital Theory
Molecular Orbital Theory The Lewis Structure approach provides an extremely simple method for determining the electronic structure of many molecules. It is a bit simplistic, however, and does have trouble predicting structures for a few molecules. Nevertheless, it gives a reasonable structure for many molecules and its simplicity to use makes it a very useful tool for chemists. A more general, but slightly more complicated approach is the Molecular Orbital Theory. This theory builds on the electron wave functions of Quantum Mechanics to describe chemical bonding. To understand MO Theory let's first review constructive and destructive interference of standing waves starting with the full constructive and destructive interference that occurs when standing waves overlap completely. When standing waves only partially overlap we get partial constructive and destructive interference. To see how we use these concepts in Molecular Orbital Theory, let's start with H2, the simplest of all molecules. The 1s orbitals of the H-atom are standing waves of the electron wavefunction. In Molecular Orbital Theory we view the bonding of the two H-atoms as partial constructive interference between standing wavefunctions of the 1s orbitals. The energy of the H2 molecule with the two electrons in the bonding orbital is lower by 435 kJ/mole than the combined energy of the two separate H-atoms. On the other hand, the energy of the H2 molecule with two electrons in the antibonding orbital is higher than two separate H-atoms. To show the relative energies we use diagrams like this: In the H2 molecule, the bonding and anti-bonding orbitals are called sigma orbitals (σ).