Toxicological Profile for Nickel
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Development of the Periodic Table and Its Consequences Citation: J
Firenze University Press www.fupress.com/substantia The Development of the Periodic Table and its Consequences Citation: J. Emsley (2019) The Devel- opment of the Periodic Table and its Consequences. Substantia 3(2) Suppl. 5: 15-27. doi: 10.13128/Substantia-297 John Emsley Copyright: © 2019 J. Emsley. This is Alameda Lodge, 23a Alameda Road, Ampthill, MK45 2LA, UK an open access, peer-reviewed article E-mail: [email protected] published by Firenze University Press (http://www.fupress.com/substantia) and distributed under the terms of the Abstract. Chemistry is fortunate among the sciences in having an icon that is instant- Creative Commons Attribution License, ly recognisable around the world: the periodic table. The United Nations has deemed which permits unrestricted use, distri- 2019 to be the International Year of the Periodic Table, in commemoration of the 150th bution, and reproduction in any medi- anniversary of the first paper in which it appeared. That had been written by a Russian um, provided the original author and chemist, Dmitri Mendeleev, and was published in May 1869. Since then, there have source are credited. been many versions of the table, but one format has come to be the most widely used Data Availability Statement: All rel- and is to be seen everywhere. The route to this preferred form of the table makes an evant data are within the paper and its interesting story. Supporting Information files. Keywords. Periodic table, Mendeleev, Newlands, Deming, Seaborg. Competing Interests: The Author(s) declare(s) no conflict of interest. INTRODUCTION There are hundreds of periodic tables but the one that is widely repro- duced has the approval of the International Union of Pure and Applied Chemistry (IUPAC) and is shown in Fig.1. -

The Periodic Table of Elements
The Periodic Table of Elements 1 2 6 Atomic Number = Number of Protons = Number of Electrons HYDROGENH HELIUMHe 1 Chemical Symbol NON-METALS 4 3 4 C 5 6 7 8 9 10 Li Be CARBON Chemical Name B C N O F Ne LITHIUM BERYLLIUM = Number of Protons + Number of Neutrons* BORON CARBON NITROGEN OXYGEN FLUORINE NEON 7 9 12 Atomic Weight 11 12 14 16 19 20 11 12 13 14 15 16 17 18 SODIUMNa MAGNESIUMMg ALUMINUMAl SILICONSi PHOSPHORUSP SULFURS CHLORINECl ARGONAr 23 24 METALS 27 28 31 32 35 40 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 POTASSIUMK CALCIUMCa SCANDIUMSc TITANIUMTi VANADIUMV CHROMIUMCr MANGANESEMn FeIRON COBALTCo NICKELNi CuCOPPER ZnZINC GALLIUMGa GERMANIUMGe ARSENICAs SELENIUMSe BROMINEBr KRYPTONKr 39 40 45 48 51 52 55 56 59 59 64 65 70 73 75 79 80 84 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 RUBIDIUMRb STRONTIUMSr YTTRIUMY ZIRCONIUMZr NIOBIUMNb MOLYBDENUMMo TECHNETIUMTc RUTHENIUMRu RHODIUMRh PALLADIUMPd AgSILVER CADMIUMCd INDIUMIn SnTIN ANTIMONYSb TELLURIUMTe IODINEI XeXENON 85 88 89 91 93 96 98 101 103 106 108 112 115 119 122 128 127 131 55 56 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 CESIUMCs BARIUMBa HAFNIUMHf TANTALUMTa TUNGSTENW RHENIUMRe OSMIUMOs IRIDIUMIr PLATINUMPt AuGOLD MERCURYHg THALLIUMTl PbLEAD BISMUTHBi POLONIUMPo ASTATINEAt RnRADON 133 137 178 181 184 186 190 192 195 197 201 204 207 209 209 210 222 87 88 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 FRANCIUMFr RADIUMRa RUTHERFORDIUMRf DUBNIUMDb SEABORGIUMSg BOHRIUMBh HASSIUMHs MEITNERIUMMt DARMSTADTIUMDs ROENTGENIUMRg COPERNICIUMCn NIHONIUMNh -

The Nickel Silvers
Copper Development Association The Nickel Silvers Design Data and Applications 1965 Please note this publication is provided as an archive copy. The information given may therefore not be current. The Nickel Silvers Design Data and Applications 1965 Copper Development Association Copper Development Association is a non-trading organisation sponsored by the copper producers and fabricators to encourage the use of copper and copper alloys and to promote their correct and efficient application. Its services, which include the provision of technical advice and information, are available to those interested in the utilisation of copper in all its aspects. The Association also provides a link between research and user industries and maintains close contact with other copper development associations throughout the world. Website: www.copperinfo.co.uk Email: [email protected] Copyright: All information in this document is the copyright of Copper Development Association Disclaimer: Whilst this document has been prepared with care, Copper Development Association can give no warranty regarding the contents and shall not be liable for any direct, indirect or consequential loss arising out of its use Contents Contents ...............................................................................................................................................................1 Introduction .........................................................................................................................................................2 -

Special-Purpose Nickel Alloys
© 2000 ASM International. All Rights Reserved. www.asminternational.org ASM Specialty Handbook: Nickel, Cobalt, and Their Alloys (#06178G) Special-Purpose Nickel Alloys NICKEL-BASE ALLOYS have a number of meet special needs. The grades considered in ganese, and copper, a 0.005% limit on iron, and unique properties, or combinations of proper- this section include the following: a 0.02% limit on carbon. This high purity re- ties, that allow them to be used in a variety of sults in lower coefficient of expansion, electri- specialized applications. For example, the high • Nickel 200 (99.6% Ni, 0.04% C) cal resistivity, Curie temperature, and greater resistivity (resistance to flow of electricity) and • Nickel 201 (99.6% Ni, 0.02% C maximum) ductility than those of other grades of nickel heat resistance of nickel-chromium alloys lead • Nickel 205 (99.6% Ni, 0.04% C, 0.04% Mg) and makes Nickel 270 especially useful for to their use as electric resistance heating ele- • Nickel 233 (see composition in table that fol- some electronics applications such as compo- ments. The soft magnetic properties of lows) nents of hydrogen thyratrons and as a substrate nickel-iron alloys are employed in electronic • Nickel 270 (99.97% Ni) for precious metal cladding. devices and for electromagnetic shielding of computers and communication equipment. Iron- Composition limits and property data on sev- eral of these grades can be found in the article nickel alloys have low expansion characteris- Resistance Heating Alloys tics as a result of a balance between thermal ex- “Wrought Corrosion-Resistant Nickels and pansion and magnetostrictive changes with Nickel Alloys” in this Handbook. -

Properties of Gold-Nickel Alloy Brazed Joints in High Temperature Materials
Properties of Gold-Nickel Alloy Brazed Joints in High Temperature Materials Professor Jakob Colbus University of Saarland, Saarbrücken, West Germany and Karl Friedrich Zimmermann Degussa, Wolfgang, West Germany The usefulness of gold-nickel alloys as brazing media in the aero- engine, electronic, nuclear power and spacecraft industries is illustrated by data on the corrosion and oxidation resistance of joints made with these alloys in various heat-resistant materials and on their resistance to fracture under the influence of static, dynamic and impact loads in tests at subzero, normal and elevated temperatures. Gold solders have been in use for many years, Brazing temperature in the range 900 to 1000°C. especially in the jewellery trade and in dentistry. Ability to make joints that retain their strength The solders used in the fabrication of jewellery and ductility at elevated temperatures. contain silver, copper, cadmium, and zinc, which are Good resistance to oxidation and corrosion. added to ensure that the colour and the gold content All these requirements can be satisfied by several of a given solder matches the corresponding charac- alloys in the gold-nickel system, whose constitutional teristics of the soldered article. The solders used in diagram is shown in Figure 1. The 18 per cent dental practice often contain other precious metals nickel-gold alloy is the most suitable one to serve as a such as iridium, palladium, and platinum introduced brazing medium. Its melting point of 950°C is the to increase their corrosion resistance, the most important property of solders of this kind. In both these applications the soldering is done in air with WEIGHT PERCENT NICKEL the aid of a gas torch and a suitable flux. -

Mechanical Properties of Electroformed Nickel Cobalt Alloys
Mechanical Properties of Electroformed Nickel Cobalt Alloys B. Stein, P. Jaeger and C. Przybyla, NiCoForm, Inc., Rochester, New York, USA Abstract Hardness was measured on ½" - thick stainless steel blocks plated with at least 0.02" of Ni-Co on a Mechanical properties of nickel-cobalt alloys (0-10% Rockwell superficial hardness tester (Wilson Co) electroformed with low internal stress in Instruments Mod. 3 JS) with a 15kg load. The sulfamate-based solutions were investigated in as average of three readings was taken for each data point deposited and heat treated conditions. It was in tensile and hardness tests. demonstrated that alloy properties can be modified by selecting deposition conditions and alloy composition. Both foil samples and hardness test blocks were Recommendations on alloy selection are made based electroformed at 1-3 A/Dm2 in two proprietary Ni-Co on application requirements. alloy electroforming chemistries, one designed for enhanced deposit hardness, the other - for high tensile The higher strength and hardness of nickel-cobalt alloy strength. Deposit internal stress was measured directly electrodeposits compared to nickel stimulates their in the plating tanks as described elsewhere [1], while increasing use in electroforming and as engineering tensile and hardness samples were being coatings. Many advanced electroformed products such electroformed. Tensile and hardness tests were as bellows, contacts, optical and nanofluidic molds can performed on samples in the as plated and heat treated only be produced consistently if the deposits’ (2 hours at the set temperature) conditions. properties are well understood and properly controlled Representative foil samples were chemically analyzed by the process engineer. -
Nickel Data Sheet
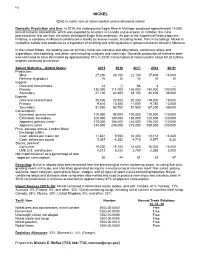
112 NICKEL (Data in metric tons of nickel content unless otherwise noted) Domestic Production and Use: In 2019, the underground Eagle Mine in Michigan produced approximately 14,000 tons of nickel in concentrate, which was exported to smelters in Canada and overseas. In October, the mine processed the first ore from the newly developed Eagle East extension. As part of the Superfund Redevelopment Initiative, a company in Missouri constructed a facility to recover metals, including nickel, from mine tailings. Nickel in crystalline sulfate was produced as a byproduct of smelting and refining platinum-group-metal ores mined in Montana. In the United States, the leading uses for primary nickel are stainless and alloy steels, nonferrous alloys and superalloys, electroplating, and other uses including catalysts and chemicals. Domestic production of stainless steel was estimated to have decreased by approximately 10% in 2019. Consumption of nickel used in alloys for jet turbine engines continued to increase. Salient Statistics—United States: 2015 2016 2017 2018 2019e Production: Mine 27,200 24,100 22,100 17,600 14,000 Refinery, byproduct W W W W W Imports: Ores and concentrates 24 (1) 64 3 — Primary 130,000 111,000 150,000 144,000 120,000 Secondary 27,100 32,300 38,100 45,100 38,000 Exports: Ores and concentrates 25,400 22,400 20,000 219,000 19,000 Primary 9,610 10,300 11,000 9,780 13,000 Secondary 51,900 63,700 51,500 67,200 49,000 Consumption: Estimated, primary metal 110,000 98,000 100,000 110,000 110,000 Estimated, secondary 120,000 130,000 130,000 120,000 120,000 Apparent, primary metal3 118,000 104,000 140,000 136,000 110,000 Apparent, total4 234,000 235,000 273,000 259,000 230,000 Price, average annual, London Metal Exchange (LME): Cash, dollars per metric ton 11,831 9,594 10,403 13,114 14,000 Cash, dollars per pound 5.367 4.352 4.719 5.977 6.30 Stocks, yearend: Consumer 19,200 15,100 14,600 16,300 16,000 LME U.S. -

Periodic Table 1 Periodic Table
Periodic table 1 Periodic table This article is about the table used in chemistry. For other uses, see Periodic table (disambiguation). The periodic table is a tabular arrangement of the chemical elements, organized on the basis of their atomic numbers (numbers of protons in the nucleus), electron configurations , and recurring chemical properties. Elements are presented in order of increasing atomic number, which is typically listed with the chemical symbol in each box. The standard form of the table consists of a grid of elements laid out in 18 columns and 7 Standard 18-column form of the periodic table. For the color legend, see section Layout, rows, with a double row of elements under the larger table. below that. The table can also be deconstructed into four rectangular blocks: the s-block to the left, the p-block to the right, the d-block in the middle, and the f-block below that. The rows of the table are called periods; the columns are called groups, with some of these having names such as halogens or noble gases. Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new, yet to be discovered or synthesized, elements. As a result, a periodic table—whether in the standard form or some other variant—provides a useful framework for analyzing chemical behavior, and such tables are widely used in chemistry and other sciences. Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table. -
![The Mechanical Properties of Some Nickel-Copper and Nickel-Copper-Carbon Alloys Below 0.35 T[Subscript M] Robert Paul Zerwekh Iowa State University](https://docslib.b-cdn.net/cover/9237/the-mechanical-properties-of-some-nickel-copper-and-nickel-copper-carbon-alloys-below-0-35-t-subscript-m-robert-paul-zerwekh-iowa-state-university-1639237.webp)
The Mechanical Properties of Some Nickel-Copper and Nickel-Copper-Carbon Alloys Below 0.35 T[Subscript M] Robert Paul Zerwekh Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1970 The mechanical properties of some nickel-copper and nickel-copper-carbon alloys below 0.35 T[subscript m] Robert Paul Zerwekh Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Materials Science and Engineering Commons Recommended Citation Zerwekh, Robert Paul, "The mechanical properties of some nickel-copper and nickel-copper-carbon alloys below 0.35 T[subscript m] " (1970). Retrospective Theses and Dissertations. 4378. https://lib.dr.iastate.edu/rtd/4378 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. 71-14,279 ZERWEKH, Robert Paul, 1939- THE MECHANICAL PROPERTIES OF SOME NICKEL- COPPER AND NICKEL-COPPER-CARBON ALLOYS BELOW Iowa. State University, Ph.D., 1970 Materials Science University Microfilms, A XEROX Company, Ann Arbor, Michigan THIS DISSERTATION HAS BEEN MICROFILMED EXACTLY AS RECEIVED THE MECHANICAL PROPERTIES OF SOME NICKEL-COPPER AND NICKEL-COPPER-CARBON ALLOYS BELOW 0.35 T_ m by Robert Paul Zerwekh A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OF PHILOSOPHY Major Subject; Metallurgy Approved ; Signature was redacted for privacy. In Cnarge ot Major worjc Signature was redacted for privacy. Signature was redacted for privacy. D( Iowa State University Ames, Iowa 1970 ii TABLE OF CONTENTS Page I. -

Cathode Process in Nickel-Cobalt Alloy Deposition from Sulfamate Electrolytes—Application to Electroforming
Cathode Process in Nickel-cobalt Alloy Deposition from Sulfamate Electrolytes—Application to Electroforming By D. Golodnitsky, N.V. Gudin & G.A. Volyanuk Electroforming is a specialized application of electroplat- -0.230 and -0.270 V vs. NHE, respectively. Accordingly, it ing for the production of finished components and unique would be expected, thermodynamically, that Ni, the more articles that cannot be made by any other method. noble metal, would deposit preferentially; however, the re- Sulfamate electrolytes for Ni-Co deposition form highly verse is found to be true. The discharge rate of the more noble efficient and stable solutions, but their wide use for component is inhibited, and this causes the appearance of the electroforming has been hampered by the fact that the less noble component at a much higher ratio in the deposit mechanism of Ni+2 and Co+2 codeposition is as yet imper- than in the electrolyte. Anomalous codeposition of binary fectly understood. Study was made of the effect of electro- iron-group alloys has been widely discussed by many inves- lyte composition and operating conditions on the Ni-Co tigators,6-20 but the mechanism is far from being understood. alloy constitution and the mechanism of its electrodepo- It was assumed that formation of the less noble metal is sition. It was shown that the Faradaic efficiency of the favored in aqueous solution and that metal hydroxides (MOH+) deposition is characterized by a complicated dependence are the important charge-transfer species. Matulis et al.11 on pH with a maximum of 98.5 percent at pH 3.5 to 3.8. -

New Methods of Cleaning up Heavy Metal in Soils and Water
ENVIRONMENTAL SCIENCE AND TECHNOLOGY BRIEFS FOR CITIZENS by M. Lambert, B.A. Leven, and R.M. Green New Methods of Cleaning Up Heavy Metal in Soils and Water Innovative solutions to an environmental problem This publication is published by the Hazard- ous Substance Research Centers as part of There are several options for treating Joplin. Here, mine spoils (locally called their Technical Outreach Services for Com- or cleaning up soils contaminated with chat) cover much of the open space in- munities (TOSC) program series of Environ- heavy metals. This paper discusses side the city, and contain high levels of mental Science and Technology Briefs for three of those methods. lead, zinc, and cadmium. Heavy metal Citizens. If you would like more information about the TOSC program, contact your re- contamination can be carried with soil gional coordinator: Introduction particles swept away from the initial At many sites around the nation, heavy areas of pollution by wind and rain. Northeast HSRC metals have been mined, smelted, or Once these soil particles have settled, New Jersy Institute of Technology Otto H. York CEES used in other industrial processes. The the heavy metals may spread into the 138 Warren St. waste (tailings, smelter slag, etc.) has surroundings, polluting new areas. Newark, NJ 07102 sometimes been left behind to pollute Cleanup (or remediation) technologies (201) 596-5846 surface and ground water. The heavy available for reducing the harmful ef- Great Plains/Rocky Mountain HSRC metals most frequently encountered in fects at heavy metal-contaminated sites Kansas State University this waste include arsenic, cadmium, include excavation (physical removal of 101 Ward Hall chromium, copper, lead, nickel, and the contaminated material), stabiliza- Manhattan, KS 66506 zinc, all of which pose risks for human tion of the metals in the soil on site, (800) 798-7796 health and the environment. -

Periodic Table P J STEWART / SCIENCE PHOTO LIBRARY PHOTO SCIENCE / STEWART J P
Periodic table P J STEWART / SCIENCE PHOTO LIBRARY PHOTO SCIENCE / STEWART J P 46 | Chemistry World | March 2009 www.chemistryworld.org Periodic change The periodic table, cherished by generations of chemists, has steadily evolved over time. Eric Scerri is among those now calling for drastic change The periodic table has become recurrences as vertical columns or something of a style icon while In short groups. remaining indispensable to chemists. In its original form The notion of chemical reactivity Over the years the table has had the periodic table was is something of a vague one. To make to change to accommodate new relatively simple. Over this idea more precise, the periodic elements. But some scientists the years, extra elements table pioneers focused on the propose giving the table a makeover have been added and the maximum valence of each element while others call for drastic changes layout of the transition and looked for similarities among to its core structure. elements altered these quantities (see Mendeleev’s More than 1000 periodic systems Some call for drastic table, p48). have been published since the table rearrangements, The method works very well for Russian chemist Dimitri Mendeleev perhaps placing hydrogen the elements up to atomic weight developed the mature periodic with the halogens. 55 (manganese) after which point system – the most fundamental A new block may be it starts to fall apart. Although natural system of classification needed when chemists there seems to be a repetition in the ever devised. (Not to mention the can make elements in highest valence of aluminium and hundreds if not thousands of new the g-block, starting at scandium (3), silicon and titanium systems that have appeared since the element 121 (4), phosphorus and vanadium (5), advent of the internet.) and chlorine and manganese (7), Such a proliferation prompts this is not the case with potassium questions as to whether some tables and iron.