Food Chemistry 129 (2011) 1001–1011
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Publication of an Application for Approval of a Minor Amendment in Accordance with the Second Subparagraph of Article 53(2)
30.8.2016 EN Official Journal of the European Union C 315/3 OTHER ACTS EUROPEAN COMMISSION Publication of an application for approval of a minor amendment in accordance with the second subparagraph of Article 53(2) of Regulation (EU) No 1151/2012 of the European Parliament and of the Council (2016/C 315/03) The European Commission has approved this minor amendment in accordance with the third subparagraph of Article 6(2) of Commission Delegated Regulation (EU) No 664/2014 (1). APPLICATION FOR APPROVAL OF A MINOR AMENDMENT Application for approval of a minor amendment in accordance with the second subparagraph of Article 53(2) of Regulation (EU) No 1151/2012 of the European Parliament and of the Council (2) ‘BRA’ EU No: PDO-IT-02128 — 18.3.2016 PDO ( X ) PGI ( ) TSG ( ) 1. Applicant group and legitimate interest Consorzio di Tutela del Formaggio BRA DOP (association for the protection of ‘Bra’ PDO cheese) Via Silvio Pellico 10 10022 Carmagnola (TO) ITALIA Tel. +39 0110565985. Fax +39 0110565989. Email: [email protected] The Consorzio di Tutela del Formaggio BRA DOP is entitled to submit an amendment application pursuant to Article 13(1) of Ministry of Agricultural, Food and Forestry Policy Decree No 12511 of 14 October 2013. 2. Member State or Third Country Italy 3. Heading in the product specification affected by the amendment(s) — Product description — Proof of origin — Production method — Link — Labelling — Other [to be specified] 4. Type of amendment(s) — Amendment to the product specification of a registered PDO or PGI to be qualified as minor in accordance with the third subparagraph of Article 53(2) of Regulation (EU) No 1151/2012, that requires no amend ment to the published single document. -

Scarica L'appendice Con L'elenco Completo Degli Edifici Dismessi
Appendice II del Quaderno 37 della Collana della Fondazione CRC Rigenerare spazi dismessi Nuove prospettive per la comunità Elenco completo dei beni dismessi rilevati in provincia di Cuneo attraverso le fonti e grazie alle segnalazioni degli enti territoriali (aggiornato al 18 giugno 2018) Nota metodologica A cura di Fondazione Fitzcarraldo Si rende disponibile l’elenco dei 449 beni dismessi in provincia di Cu- neo rilevati attraverso le fonti disponibili e grazie alle segnalazioni dirette degli enti territoriali nell’ambito della mappatura realizzata per il Quaderno 37 Rigenerare spazi dismessi. Nuove prospettive per la comunità, pro- mosso dalla Fondazione CRC e realizzato da Fondazione Fitzcarraldo. Di seguito si specificano alcune considerazioni relative alla metodolo- gia adottata e alcune indicazioni utili alla consultazione dell’elenco. La scelta effettuata per disporre di una prima ricognizione di parte del patrimonio dismesso cuneese si è concretata, da un lato, in una ricerca e una lettura dei dati estrapolati dalle poche fonti disponibili e, dall’altro, nella raccolta di segnalazioni dirette da parte degli enti territoriali. Una mappatura efficace e puntuale in grado di fotografare dal punto di vista quantitativo e localizzativo tutte le opportunità e gli edifici in stato di abbandono e di sottoutilizzo in un territorio così vasto come la provincia di Cuneo non può prescindere da sopralluoghi mirati in tutti i 250 comuni del territorio. Per questo è più realistico definire questa parte della ricerca come una ricognizione “a maglie larghe” di un immenso patrimonio di- smesso che comprende una moltitudine di proprietari differenti. Le diverse fonti oggetto della presente ricognizione individuano beni le cui condizioni effettive non sono direttamente verificate, non essendo stati effettuati i sopralluoghi. -

Cuneo, 10 Dicembre 2020 Oggetto: COMUNICATO STAMPA AL VIA LA
Cuneo, 10 dicembre 2020 Oggetto: COMUNICATO STAMPA AL VIA LA NUOVA DISTRIBUZIONE DEI BUONI SPESA NEI COMUNI DI ACCEGLIO, ARGENTERA, BEINETTE, BERNEZZO, BORGO SAN DALMAZZO, CANOSIO, CARAGLIO, CARTIGNANO, CELLE DI MACRA, DRONERO, MACRA, MARGARITA, MARMORA, MOIOLA, MONTANERA, MONTEMALE, MONTEROSSO GRANA, MOROZZO, PRADLEVES, ROBILANTE, ROCCABRUNA, ROCCASPARVERA, ROCCAVIONE, SAMBUCO, VALDIERI, VALGRANA, VIGNOLO Prenderà avvio lunedì 14 dicembre la raccolta delle domande per l’attribuzione dei buoni spesa istituiti dal Governo ai cittadini residenti nei Comuni di Acceglio, Argentera, Beinette, Bernezzo, Borgo San Dalmazzo, Canosio, Caraglio, Cartignano, Celle Macra, Dronero, Macra, Margarita, Marmora, Moiola, Montanera, Montemale, Monterosso Grana, Morozzo, Pradleves, Robilante, Roccabruna, Roccasparvera, Roccavione, Sambuco, Valdieri, Valgrana e Vignolo. Come noto, il Governo ha stanziato un nuovo fondo da 400 milioni di euro per iniziative di solidarietà alimentare, come già avvenuto in primavera, destinato ad aiutare le persone e le famiglie più esposte agli effetti economici dell’emergenza epidemiologica in corso. Ai 53 Comuni facenti parte del Consorzio Socio Assistenziale del Cuneese sono stati destinati complessivamente 930 mila euro: alcuni di essi, hanno rinnovato la delega al Consorzio per la raccolta delle istanze e la valutazione tecnico professionale delle stesse. I 27 Comuni che hanno aderito alla gestione congiunta del Consorzio hanno una dotazione complessiva di 297.086 mila euro, a fronte di una popolazione di 51.469 abitanti. I cittadini residenti in uno dei 27 Comuni che vogliano presentare domanda, potranno contattare telefonicamente i punti di raccolta messi a disposizione dal Consorzio, nell’orario ore 9-13 dal lunedì al venerdì. Per i Comuni di Beinette, Margarita, Montanera, Tel. 0171 334 666 Morozzo Per i Comuni di Caraglio, Montemale, Monterosso Tel. -

Aa Aa Comune Di Montemale Di Cuneo 2016-08-29 55910 Pdf
REGIONE PIEMONTE BU36 08/09/2016 Comune di Montemale di Cuneo (Cuneo) Recepimento atto di indirizzo dell’Unione Montana Valle Grana per la formazione di una Commissione Edilizia unica gestita in forma associata a livello di Unione Montana per i Comuni di Valgrana, Montemale di Cuneo, Monterosso Grana, Pradleves e Castelmagno. Modifica del Regolamento Edilizio Comunale. … Omissis … IL CONSIGLIO COMUNALE …..Omissis…… 1) Di prendere atto che con propria precedete deliberazione è stato approvato lo schema di convenzione per la gestione associata dello Sportello Unico per l’edilizia privata e l’urbanistica istituito ai sensi dell’art. 5 comma 1 del D.P.R. 380/2001 tra i Comuni di Valgrana, Montemale di Cuneo, Monterosso di Cuneo, Pradleves, Castelmagno e l'Unione Montana Valle Grana; 2) Di esprimere parere favorevole alla formazione di una Commissione Edilizia Unica per i Comuni di Valgrana, Montemale, Monterosso Grana, Pradleves e Castelmagno gestita in forma associata nell’ambito dello Sportello Unico per l’edilizia; 3) di modificare l’art. 2, che disciplina la “Formazione della Commissione Edilizia”, l’art. 3 che definisce le “Attribuzioni della Commissione Edilizia” e l’art. 4 che norma il “Funzionamento della Commissione Edilizia” del Regolamento Edilizio comunale del Comune di Pradleves stabilendo la sostituzione dei seguenti commi: Art. 2 - Formazione della Commissione Edilizia Il comma 1. La Commissione Edilizia è l’organo tecnico consultivo comunale nel settore urbanistico ed edilizio. è sostituito come segue: comma 1. La Commissione Edilizia è l’organo tecnico consultivo dell’Unione Montana nel settore urbanistico ed edilizio per i comuni che aderiscono alla gestione associata dello Sportello Unico per l’edilizia. -
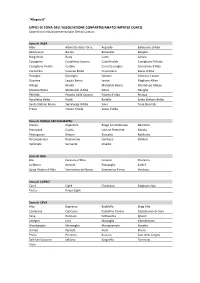
Zone Del Sistema Confartigianato Cuneo -> Comuni
“Allegato B” UFFICI DI ZONA DELL’ASSOCIAZIONE CONFARTIGIANATO IMPRESE CUNEO Zone e loro limitazione territoriale. Elenco Comuni. Zona di ALBA Alba Albaretto della Torre Arguello Baldissero d’Alba Barbaresco Barolo Benevello Bergolo Borgomale Bosia Camo Canale Castagnito Castelletto Uzzone Castellinaldo Castiglione Falletto Castiglione Tinella Castino Cerretto Langhe Corneliano d’Alba Cortemilia Cossano Belbo Cravanzana Diano d’Alba Feisoglio Gorzegno Govone Grinzane Cavour Guarene Lequio Berria Levice Magliano Alfieri Mango Montà Montaldo Roero Montelupo Albese Monteu Roero Monticello d’Alba Neive Neviglie Perletto Pezzolo Valle Uzzone Piobesi d’Alba Priocca Rocchetta Belbo Roddi Rodello Santo Stefano Belbo Santo Stefano Roero Serralunga d’Alba Sinio Tone Bormida Treiso Trezzo Tinella Vezza d’Alba Zona di BORGO SAN DALMAZZO Aisone Argentera Borgo San Dalmazzo Demonte Entracque Gaiola Limone Piemonte Moiola Pietraporzio Rittana Roaschia Robilante Roccasparvera Roccavione Sambuco Valdieri Valloriate Vernante Vinadio Zona di BRA Bra Ceresole d’Alba Cervere Cherasco La Morra Narzole Pocapaglia Sanfrè Santa Vittoria d’Alba Sommariva del Bosco Sommariva Perno Verduno Zona di CARRÙ Carrù Cigliè Clavesana Magliano Alpi Piozzo Rocca Cigliè Zona di CEVA Alto Bagnasco Battifollo Briga Alta Camerana Caprauna Castellino Tanaro Castelnuovo di Ceva Ceva Garessio Gottasecca Igliano Lesegno Lisio Marsaglia Mombarcaro Mombasiglio Monesiglio Montezemolo Nucetto Ormea Paroldo Perlo Priero Priola Prunetto Roascio Sale delle Langhe Sale San Giovanni Saliceto -

Second Report Submitted by Italy Pursuant to Article 25, Paragraph 1 of the Framework Convention for the Protection of National Minorities
Strasbourg, 14 May 2004 ACFC/SR/II(2004)006 SECOND REPORT SUBMITTED BY ITALY PURSUANT TO ARTICLE 25, PARAGRAPH 1 OF THE FRAMEWORK CONVENTION FOR THE PROTECTION OF NATIONAL MINORITIES (received on 14 May 2004) MINISTRY OF THE INTERIOR DEPARTMENT FOR CIVIL LIBERTIES AND IMMIGRATION CENTRAL DIRECTORATE FOR CIVIL RIGHTS, CITIZENSHIP AND MINORITIES HISTORICAL AND NEW MINORITIES UNIT FRAMEWORK CONVENTION FOR THE PROTECTION OF NATIONAL MINORITIES II IMPLEMENTATION REPORT - Rome, February 2004 – 2 Table of contents Foreword p.4 Introduction – Part I p.6 Sections referring to the specific requests p.8 - Part II p.9 - Questionnaire - Part III p.10 Projects originating from Law No. 482/99 p.12 Monitoring p.14 Appropriately identified territorial areas p.16 List of conferences and seminars p.18 The communities of Roma, Sinti and Travellers p.20 Publications and promotional activities p.28 European Charter for Regional or Minority Languages p.30 Regional laws p.32 Initiatives in the education sector p.34 Law No. 38/2001 on the Slovenian minority p.40 Judicial procedures and minorities p.42 Database p.44 Appendix I p.49 - Appropriately identified territorial areas p.49 3 FOREWORD 4 Foreword Data and information set out in this second Report testify to the considerable effort made by Italy as regards the protection of minorities. The text is supplemented with fuller and greater details in the Appendix. The Report has been prepared by the Ministry of the Interior – Department for Civil Liberties and Immigration - Central Directorate for Civil Rights, Citizenship and Minorities – Historical and new minorities Unit When the Report was drawn up it was also considered appropriate to seek the opinion of CONFEMILI (National Federative Committee of Linguistic Minorities in Italy). -

Itinerario Alternativo E Sostenibile Per La Messa in Rete Delle Tipicità Della Bassa Valle Grana” ______
PTI CUNEO E LE SUE VALLI Il polo agroalimentare e agroindustriale di qualità “Itinerario alternativo e sostenibile per la messa in rete delle tipicità della bassa Valle Grana” ___________________________________________________________________________ STUDIO DI FATTIBILITA’ Codice intervento: TUT-7-P Denominazione: Itinerario alternativo e sostenibile per la messa in rete delle tipicità della bassa Valle Grana Linea progettuale di riferimento: PRIORITA’ III-RIQUALIFICAZIONE TERRITORIALE III.6 Promozione e valorizzazione dei sistemi turistico-culturali; tutela e valorizzazione del paesaggio Asse strategico di intervento: TUTELA INTEGRITA’ E VALORIZZAZIONE PATRIMONIO STORICO RURALE ALPINO Localizzazione: Comuni di Caraglio, Cervasca, Vignolo, Bernezzo, Valgrana e Monterosso. Intervento strategico: SI 1.QUADRO CONOSCITIVO 1.1 Descrizione dell’intervento e del contesto di riferimento L’iniziativa si colloca in bassa Valle Grana e coinvolge direttamente sei differenti Comuni: Caraglio, Cervasca, Vignolo, Bernezzo, Valgrana e Monterosso. Situata nella parte sud occidentale della Regione Piemonte, la valle costituisce una delle undici Comunità Montane della Provincia di Cuneo, incassata fra le due maggiori Valli Stura e Maira con un ampio sbocco sulla pianura cuneese, il quale va gradualmente restringendosi nel risalire la vallata. Il suo territorio, tipicamente prealpino, è incassato e presenta fiancate di moderata altitudine e declivi dolci, folti boschi, nella parte inferiore, e ampi pascoli nella conca che si apre nell’alto bacino attorno al Comune di Castelmagno. Dal punto di vista amministrativo, il territorio della Comunità Montana raggruppa nove Comuni: Bernezzo, Caraglio, Castelmagno, Cervasca, Montemale, Monterosso Grana, Pradleves, Valgrana, Vignolo. Il capoluogo, Caraglio, sorge a 583 metri s.l.m. ed è l’unico Comune, tra i nove della Comunità Montana, non classificato montano, sia secondo la suddivisione ISTAT per fasce altimetriche (che lo definisce Comune di pianura), sia secondo la L.R. -

Piemonte T a Z Z I N a G R O
La Strategia Nazionale per le Aree Interne e i nuovi assetti istituzionali AREA INTERNA O VALLI GRANA E MAIRA V I REGIONE PIEMONTE T A Z Z I N A G R O A E R A ' D R E I S S O D Nota introduttiva Le Aree Interne rappresentano una ampia parte del Paese. Si tratta di aree significativamente distanti dai centri di offerta di servizi essenziali (quali istruzione, salute e mobilità) ma ricche di importanti risorse ambientali e culturali, fortemente diversificate per natura e per processi di antropizzazione. Un quarto della popolazione italiana occupa queste aree, con un’estensione territoriale che supera il sessanta per cento del totale della superficie nazionale e interessa oltre quattromila comuni. Il Piano Nazionale di Riforma (PNR) ha individuato e messo in atto una Strategia che ha come obiettivo non solo la ripresa demografica, ma anche un miglioramento qualitativo di vita promuovendo per queste aree uno sviluppo intensivo (benessere e inclusione sociale) ed estensivo (lavoro e utilizzo di risorse locali) attraverso fondi ordinari della Legge di Stabilità e Fondi comunitari. La Strategia Nazionale per le Aree Interne, che coinvolge un quarto dei comuni classificati come aree interne, ha individuato e selezionato 72 aree progetto, ricadenti in ambiti territoriali omogenei, distribuite su tutto il territorio nazionale. Per esse si è avviato un processo di crescita e coesione territoriale. Il Dossier d’area organizzativo è un documento di sintesi (analitica e documentale) su alcune condizioni strutturali dell’area e sulle scelte che i comuni hanno effettuato per rafforzare la loro capacità di gestire i servizi pubblici locali e i progetti previsti dalla Strategia. -

Medie Radon Provincia Cuneo 2017
Provincia Comune media radon al piano terra (Bq/m 3) Cuneo Acceglio 133 Cuneo Aisone 149 Cuneo Alba 99 Cuneo Albaretto della torre 79 Cuneo Alto 498 Cuneo Argentera 216 Cuneo Arguello 79 Cuneo Bagnasco 112 Cuneo Bagnolo Piemonte 135 Cuneo Baldissero d'Alba 105 Cuneo Barbaresco 89 Cuneo Barge 145 Cuneo Barolo 85 Cuneo Bastia mondovi' 108 Cuneo Battifollo 96 Cuneo Beinette 160 Cuneo Bellino 80 Cuneo Belvedere Langhe 79 Cuneo Bene Vagienna 148 Cuneo Benevello 79 Cuneo Bergolo 81 Cuneo Bernezzo 102 Cuneo Bonvicino 79 Cuneo Borgo San Dalmazzo 133 Cuneo Borgomale 79 Cuneo Bosia 87 Cuneo Bossolasco 79 Cuneo Boves 140 Cuneo Bra 146 Cuneo Briaglia 82 Cuneo Briga Alta 125 Cuneo Brondello 120 Cuneo Brossasco 118 Cuneo Busca 148 Cuneo Camerana 83 Cuneo Camo 80 Cuneo Canale 107 Cuneo Canosio 130 Cuneo Caprauna 602 Cuneo Caraglio 63 Cuneo Caramagna Piemonte 157 Cuneo Carde' 155 Cuneo Carru' 147 Cuneo Cartignano 116 Cuneo Casalgrasso 154 Cuneo Castagnito 92 Cuneo Casteldelfino 90 Cuneo Castellar 143 Cuneo Castelletto Stura 154 Cuneo Castelletto Uzzone 81 Cuneo Castellinaldo 98 Cuneo Castellino Tanaro 85 Cuneo Castelmagno 96 Cuneo Castelnuovo di Ceva 99 Cuneo Castiglione Falletto 94 Cuneo Castiglione Tinella 81 Cuneo Castino 81 Cuneo Cavallerleone 161 Cuneo Cavallermaggiore 160 Cuneo Celle di Macra 73 Cuneo Centallo 159 Cuneo Ceresole d'Alba 151 Cuneo Cerretto Langhe 79 Cuneo Cervasca 142 Cuneo Cervere 151 Cuneo Ceva 105 Cuneo Cherasco 140 Cuneo Chiusa di Pesio 147 Cuneo Ciglie' 98 Cuneo Cissone 79 Cuneo Clavesana 94 Cuneo Corneliano d'Alba 104 Cuneo -

“ISTITUTO STORICO DELLA RESISTENZA E DELLA SOCIETA' CONTEMPORANEA in PROVINCIA DI CUNEO DANTE LIVIO BIANCO” Codice Fiscale
“ISTITUTO STORICO DELLA RESISTENZA E DELLA SOCIETA’ CONTEMPORANEA IN PROVINCIA DI CUNEO DANTE LIVIO BIANCO” Codice fiscale e Partita IVA: C.F. 80017990047 Sede Cuneo-largo Barale 11 Telefono-fax-e.mail 0171/444830 0171/444840 [email protected] Sito internet www.istitutoresistenzacuneo.it Scopo sociale L’Istituto non ha finalità di lucro e si propone di: a) reperire e curare in modo da consentire la consultazione da parte di ricercatori, studenti e di chi ne abbia interesse la raccolta delle testimonianze, dei documenti, delle pubblicazioni e di tutto ciò che riguarda la storia contemporanea con particolare riferimento al fascismo, all’antifascismo, al movimento e alla guerra di liberazione; b) promuovere la conoscenza anche attraverso l’organizzazione di seminari, convegni, l’attribuzione di borse di studio e di ricerca, della formazione e sviluppo del nostro Stato democratico e repubblicano e del suo ordinamento costituzionale con particolare riferimento ai movimenti politici e militari della lotta di liberazione; c) contribuire allo sviluppo e all’aggiornamento dell’insegnamento della storia nelle scuole anche collaborando alla predisposizione e attuazione di programmi didattici intesi a diffondere nelle scuole la conoscenza della storia contemporanea; d) collaborare per la realizzazione di iniziative coerenti con le suddette finalità con università, istituti culturali, centri di ricerca, scuole, Enti locali facendo ricorso anche ad apposite convenzioni. Costituzione 14 aprile 1964 Data termine 13 aprile 2063 Tipologia Consorzio tra Enti locali Capitale sociale Non presente Quota di partecipazione del Comune di Roccavione: 0,382 % Risultato finanziario 2013 : € 38.743,53 Risultato finanziario 2014 : € 46.634,99 Risultato finanziario 2015 : € 31.087,44 Oneri gravanti sul bilancio comunale (anno 2015) € 347,86 “C.E.C.”- CONSORZIO ECOLOGICO CUNEESE Codice fiscale e Partita IVA: C.F. -

Schede Inglesi:Formaggi
cheeses Castelmagno PDD (Protected Denomination of Origin) cheese is to be found on the market in 2 typologies: “Prodotto della Montagna” and “d’Alpeggio”. Both are cylindrical with flat sides of the diameter of 15/25cm; the rim is about 12/20cm; the weight of a form is between 2 and 7kg; appearance: it appears with a non-eatable-look-like scab, thin and smooth which, the more the cheese ripens, becomes thicker and more rugged, with a yellow-reddish colour later conducive to grey; the paste has a friable structure at the beginning of the ripening, more compact when more ripened; colour: pearly-white or ivory-white at the beginning of the ripening, ochre-yellow with possible blue-greenish veining inside PRODUCT the more it ripens (also obtained by piercing the forms through the traditional technique, manual with long shackles); taste: fine, delicate and moderately salted at the beginning of ripening; more tasty when more ripened; maturing: at least 60 days. Castelmagno PDO cheese can only be produced in Castelmagno, Pradleves, Monterosso Grana, in the province of Cuneo. The milk used to produce it, must comes from those aereas. ITALIA PIEMONTE Torino Roma Torino PRODUCTION ZONE The logo is composed by a stylized “C” with sketches of alpine peaks on the top and in the middle an elliptic form of carved cheese. The Castelmagno cheese, if entirely producted over 600m a.s.l., carries on its label the words “prodotto della montagna”(“mountain product”) (the words on the label are in blue); if the production of milk and the cheese cheese production take place above 1000m a.s.l., the cheese carries on its label the words “di alpeggio” (“of MARK mountain pasture”) (the words on the label are in green). -

Scarica L'appendice Del Quaderno 37
Appendice del Quaderno 37 della Collana della Fondazione CRC Rigenerare spazi dismessi Nuove prospettive per la comunità Elenco dei beni dismessi segnalati dagli enti territoriali (aggiornato al 18 giugno 2018) Nota alla lettura Si rende disponibile l’elenco dei 127 beni dismessi in provincia di Cu- neo segnalati dagli enti territoriali nell’ambito della mappatura realizzata per il Quaderno 37, promosso dalla Fondazione CRC e realizzato da Fon- dazione Fitzcarraldo. Si precisa che l’elenco costituisce la parte di una rico- gnizione più ampia e complessa, frutto di un censimento di edifici dismessi a partire da un gruppo eterogeneo di fonti primarie e secondarie. L’elenco che segue rappresenta uno degli esiti più rilevanti del più am- pio lavoro di ricognizione poiché deriva da segnalazioni dirette pervenute prevalentemente da enti territoriali (comuni, unioni montane, ma anche iscritti all’Ordine degli architetti della provincia di Cuneo e Politecnico di Torino) in risposta a una call specifica indetta da Fondazione Fitzcarraldo nell’ambito della ricerca. Si tratta dunque di informazioni raccolte ad hoc, pertinenti e aggiornate al 18 giugno 2018. Il documento che segue, dunque, non intende essere esaustivo di tutti i beni dismessi disponibili sul territorio provinciale, per la complessità e ricchezza del patrimonio esistente e per l’ampia varietà delle fonti dispo- nibili. Rappresenta invece il frutto di segnalazioni dirette degli enti territo- riali − verificate dagli autori della ricerca − di edifici di proprietà pubblica considerati