Based Fisheries Management on Coral Reef Communities of American Samoa
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Balancing the Tides
JoAnna Poblete Lorem ipsum dolor sit amet, consectetur adipiscing elit. Nam auctor faucibus odio, a scelerisque magna sollicitudin ac. Cras condimentum lacus elementum massa pharetra malesuada. Fusce ac eros varius, viverra urna scelerisque, egestas neque. Fusce tellus arcu, euismod ut rutrum faucibus, elementum quis sem. Fusce auctor sollicitudin lacus, sed sagittis libero tincidunt ac. Praesent vestibulum Balancing tellus a massa ultrices, et mattis augue ultricies. Aliquam at turpis a nisl portti- tor rhoncus id a lacus. Integer a risus eu sapien porta vestibulum. Aenean posu- ere non diam a posuere. Duis laoreet congue tellus, posuere aliquam leo facilisis quis. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Orci varius natoque penatibus et magnis dis parturient montes, nascetur ridiculus mus. Morbi mattis the Tides consequat lacus, ut aliquet dolor accumsan non. Proin suscipit molestie convallis. Vestibulum ante ipsum primis in faucibus orci luctus et ultrices posuere cubilia Curae; Proin quam justo, feugiat non accumsan et, congue sit amet mi. Marine Practices in Vestibulum consectetur mauris et nisl ultrices, eget hendrerit dui vestibulum. Balancing the Mauris gravida sodales nulla eu mattis. Nam tincidunt eu ante vel euismod. Ali- American Sāmoa quam laoreet lacus quis tellus efficitur, vitae porttitor leo pulvinar. Integer dapi- bus gravida quam quis vehicula. Nulla metus nulla, suscipit in porta non, fermen- tum eget urna. Integer quis turpis felis. Sed finibus sem non elementum volutpat. Phasellus pulvinar orci tortor, sed congue elit pharetra eu. Curabitur sodales, dui a luctus semper, metus erat iaculis risus, ut facilisis leo mi vitae lorem. Pellentesque elementum dui ex, in pulvinar nibh maximus ut. -

Still Living Without the Basics in the 21St Century
rcap_cover_final 6/2/04 2:21 PM Page 1 Still Living Without the Basics in the 21st Century Analyzing the Availability of Wa t e r and Sanitation Services in the United States Rural Community Assistance Partnership Still Living Without the Basics Table of Contents Foreword.............................................................................................................................................................1 Executive Summary...........................................................................................................................................3 Still Living Without the Basics in the 21st Century ......................................................................................3 Still Living Without the Basics: Analyzing the Availability of Water and Sanitation Services in the United States.......................................................................................................................................................7 Introduction........................................................................................................................................................7 Methodological Layout of the Study...............................................................................................................8 Part I of the Analysis......................................................................................................................................8 Part II of the Analysis....................................................................................................................................9 -

American Samoa
Coral Reef Habitat Assessment for U.S. Marine Protected Areas: U.S. Territory of American Samoa National Oceanic and Atmospheric Administration NOAA’s National Ocean Service Management & Budget Office Special Projects February 2009 Project Overview About this Effort NCCOS Benthic Habitat Mapping Effort The United States Coral Reef Task Force (USCRTF), in both its National The National Oceanic and Atmospheric Administration (NOAA) National Action Plan to Conserve Coral Reefs (2000) and its National Coral Reef Ocean Service (NOS) initiated a coral reef research program in 1999 to Action Strategy (2002), established a key conservation objective of pro- map, assess, inventory, and monitor U.S. coral reef ecosystems (Monaco tecting at least 20% of U.S. coral reefs and associated habitat types in et al. 2001). These activities were implemented in response to require- no-take marine reserves. NOAA’s Coral Reef Conservation Program has ments outlined in the Mapping Implementation Plan developed by the Map- been supporting efforts to assess current protection levels of coral reefs ping and Information Synthesis Working Group (MISWG) of the Coral Reef within Marine Protected Areas (MPAs) and quantify the area of U.S. coral Task Force (CRTF) (MISWG 1999). NOS’s National Centers for Coastal reef ecosystems protected in no-take reserves. The official federal defini- Ocean Science (NCCOS) Biogeography Team was charged with the de- tion of an MPA, signed into law by Executive Order 13158, is “any area of velopment and implementation of a plan to produce comprehensive digital the marine environment that has been reserved by federal, state, tribal, coral-reef ecosystem maps for all U.S. -

Recovery from Disturbance
Results of the Territorial Monitoring Program of American Samoa for 2008, Benthic Section. By Douglas Fenner, Ph.D. Coral Reef Monitoring Ecologist Department of Marine & Wildlife Resources (DMWR) American Samoa 2009 Report to DMWR, the Coral Reef Advisory Group (CRAG), and NOAA Supported by a NOAA Coral Reef Monitoring Grant, part of the Coral Reef Initiative 1 Contents Acknowledgements 2 Abstract 3 Methods 6 Results 10 Reef Slopes 11 Individual Sites 18 Corals in Transects 22 Lifeforms 22 Genera 29 Species 36 Species Diversity 42 Reef Flats 46 Outer Flats 46 Trends at Individual Sites 50 Corals on outer reef flats 53 Lifeforms 53 Genera 56 Species 58 Inner Reef Flats 61 Individual Sites 64 Comparisons 67 Depth and Zonation 69 Coral Lifeforms 75 Genera 77 Species 79 Invertebrates 83 Water Clarity 93 Non-Point Pollution 95 Baselines 98 Crown-of-thorns 98 Red Tides in the Harbor 99 Seagrass 100 Major Disturbances 100 Table Coral Recruitment 101 Cloth Rags 104 Species 104 References 106 Acknowledgements Thank you to boat captain Mika Letuane. Thanks also to Ekueta Schuster for filling tanks and other support, and for DMWR enforcement for the use of their giant pickup to 2 pull the boat over the very steep pass to the boat ramp at Fagasa on the north side of Tutuila. Special thanks go to the Fagatele Bay National Marine Sanctuary, for the use of the Sanctuary boat when the DMWR boat was not available. Abstract Benthic communities remain relatively healthy with moderate live coral cover, low macroalgae and no recently dead coral, and a small increase in live coral cover in 2008. -

“Food Desert” American Samoa Assessing Food Desert at School Locations
“Food Desert” American Samoa Assessing food desert at school locations. Molly Asifoa-Lagai Food Policy Analyst Community and Natural Resources Division American Samoa Community College July 2012 ACKNOWLEDGMENT Sincere gratitude is hereby extended to the following that assisted and helped with this report: Tapa’au Dr. Daniel Aga, ASCC-CNR Dean & Director Aufa’i Ropeti Apulu Areta, ASCC-CNR Associate Director/Extension Coordinator Dr. Don Vargo, ASCC-CNR Research Coordinator Emma Apatu, East Tennessee State Doctoral Student Kasie Richards, East Tennessee State Doctoral Student Neil Gurr, ASCC-CNR GIS Specialist Tipisone Fa’alogo, ASCC-CNR Media Specialist Rocky Mane, ASCC-CNR Media Technician Mary Scanlan, ASCC-CNR Research Assistant All ASCC-CNR Staff This work has been partially funded by Agricultural Development in the American Pacific project (ADAP)- NIFA Grant #2009-38826-19927. Financial support was also provided by a US Department of Agriculture Hatch grant, CRIS Accession No. 0212428, administered by the American Samoa Community College. 2 Executive Summary American Samoa is an unincorporated US territory in the South Pacific. It consists of the Tutuila and the Manua islands. The population is estimated at 70,000. This is an island nation that is currently having an increasing problem with obesity. The US had done studies, research and implemented programs to help prevent the increasing rate of obesity. The data from studies in the US have shown one in five children suffer from child obesity. It was estimated in 2009 that 55.6% of American Samoan schoolchildren were either overweight or obese, which is more than half of the children population in the territory. -

Summary of Ground-Water Data for Tutuila and Aunuu, American Samoa, for October 1987 Through September 1997
U.S. Department of the Interior Summary of Ground-Water Data for Tutuila and Aunuu, American Samoa, for October 1987 Through September 1997 U.S. GEOLOGICAL SURVEY Open-File Report 99-252 OCEAN Bay Prepared In cooperation with the AMERICAN SAMOA ENVIRONMENTAL PROTECTION AGENCY Summary of Ground-Water Data for Tutuila and Aunuu, American Samoa, for October 1987 Through September 1997 By Scot K. Izuka U.S. GEOLOGICAL SURVEY Open-File Report 99-252 Prepared in cooperation with the AMERICAN SAMOA ENVIRONMENTAL PROTECTION AGENCY Honolulu, Hawaii 1999 U.S. DEPARTMENT OF THE INTERIOR BRUCE BABBITT, Secretary U.S. GEOLOGICAL SURVEY Charles G. Groat, Director The use of firm, trade, and brand names in this report is for identification purposes only and does not constitute endorsement by the U.S. Geological Survey. For additional information write to: Copies of this report can be purchased from: District Chief U.S. Geological Survey U.S. Geological Survey Branch of Information Services 677 Ala Moana Blvd., Suite 415 Box 25286 Honolulu, HI 96813 Denver, CO 80225-0286 CONTENTS Abstract.......................................................................................... 1 Introduction....................................................................................... 1 Setting...................................................................................... 2 Acknowledgments ............................................................................ 2 Rainfall ......................................................................................... -

ALOFAU Village 14.263453S 170.619074W 170.591294W
14.263453S CENSUS 2000 BLOCK MAP: ALOFAU Village 14.263453S 170.619074W 170.591294W LEGEND SYMBOL NAME STYLE INTERNATIONAL AIR (FEDERAL) Trust Land / Home Land OTSA / TDSA / ANVSA AMERICAN INDIAN TRIBAL SUBDIVISION AIR (State) SDAISA Masefau ANRC 54500 Masausi 53700 MINOR CIVIL DIV. / CCD 1 Consolidated City Place within Subject Entity Incorporated Place / CDP 1 Place outside of Subject Entity Incorporated Place / CDP 1 Census Tract 2 ABBREVIATION REFERENCE: AIR = American Indian Reservation; Trust Land = Off−Reservation Trust Land; Home Land = Hawaiian Home Land; OTSA = Oklahoma Tribal Statistical Area; TDSA = Tribal Designated Statistical Area; ANVSA = Alaska Native Village Statistical Area; SDAISA = State Designated American Indian Statistical Area; ANRC = Alaska Native Regional Corporation; CDP = Census Designated Place; Minor Civil Div. (MCD) = Minor Civil Division; CCD = Census County Division FEATURES Sa'ilele 66500 Aoa 12100 VAIFANUA CO 86500 Military Inset Out Area Where international, state, and/or county boundaries coincide, the map shows the boundary symbol for only the highest−ranking of these boundaries. 1 A ' * ' following a place name indicates that the place is coextensive with a MCD, A ' ° ' indicates that the place is also a false MCD; the false MCD name is not shown. 2 A ' * ' following a block number indicates that the block number is repeated elsewhere in the block. SA'OLE CO 67300 Pagai 62230 SUA CO 72100 3007 Faga'itua 23300 3000 3006 Tutuila Island Fagaitua Bay 3005 Alofau SA'OLE CO 07300 67300 3002 3001 Amouli 10500 9502 3004 3003 Key to Adjacent Sheets 1 SHEET 1 OF 1 PARENT SHEET Amaua 09700 TOTAL SHEETS: 1 (Index 0; Parent 1; Inset 0) 14.290360S 14.290360S 170.619074W 170.591294W N ENTITY TYPE: Incorporated Place .2 .1 0 .2 mi W E NAME: Alofau village (07300) ST: American Samoa (60) S .3 .2 .1 0 .3 km CO: Eastern Dis (010) All legal boundaries and names are as of January 1, 2000. -

Census of American Samoa
FOURTEENTH CENSUS OF" THE UNITED STATES: 1920 DEPARTMENT BUREAU OF THE CENSUS OF OOM ME:ROE BULLETIN SAM, L ROGERS, Du•eCToR CENSUS OF AMERICAN SAMOA INTRODUCTION. This bulletin gives the results of the census of west coast, affords a.n excellent anchorage for vessels American Samoa, which, as required by the act of during the southeast tra.de winds. March 3, 1919, providing for the Fourteenth and sub The island of Olosega lies 3} miles northwest of sequent decennial censuses of the United States, was Manua. It is separated from the island of Ofu by a taken by the governor of American Samoa in accord narrow and shallow p&'lsage. Both of these islands ance with plans prescribed by the Director of the arc mount,ainous, and their combined area is about Census. 2 square miles. Geography.-The groui) of South Pacific islands Clima.te.-As the Samoa.<\ lie wholly within tho known as American Samoa lie in latitude 14° 11' to South Torrid Zone, the. climate is tropical. During 14° 23' south andinlongitu(le 169° 29' to 170° 52' west the summer months, December to February, the of Greenwich. A clearer idea of their location may be temperature is highest, and during the winter months, gained from the statement that the distance f~om June to August, it is coolest. The highest tempera Tutuila, the largest island of the American Samoas, ture is about 88° and the lowest about 70°. Hurri to San Francisco is 4,160 nautical miles; to Honolulu, canes occasionally visit these islands. 'The rainy season 2,263 nautical miles; an4 to Sidney, Australia, 2,354 extends from December to :March, the greatest average nautical miles. -
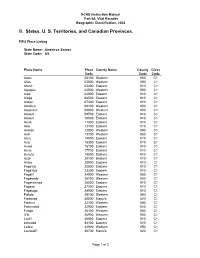
American Samoa State Code: AS
NCHS Instruction Manual Part 8A, Vital Records Geographic Classification, 2004 II. States, U. S. Territories, and Canadian Provinces. FIPS Place Listing State Name: American Samoa State Code: AS Place Name Place County Name County Class Code Code Code Aasu 00100 Western 050 C1 Afao 02500 Western 050 C1 Afono 03300 Eastern 010 C1 Agugulu 04500 Western 050 C1 Alao 04900 Eastern 010 C1 Alega 06500 Eastern 010 C1 Alofau 07300 Eastern 010 C1 Amaluia 08100 Western 050 C1 Amanave 08900 Western 050 C1 Amaua 09700 Eastern 010 C1 Amouli 10500 Eastern 010 C1 Anua 11300 Eastern 010 C1 Aoa 12100 Eastern 010 C1 Aoloau 12800 Western 050 C1 Asili 13700 Western 050 C1 Atu'u 14500 Eastern 010 C1 Aua 15300 Eastern 010 C1 Auasi 16100 Eastern 010 C1 Aumi 17700 Eastern 010 C1 Aunu'u 18500 Eastern 010 C1 Auto 20100 Eastern 010 C1 Avaio 20900 Eastern 010 C1 Faga'alu 22500 Eastern 010 C1 Faga'itua 23300 Eastern 010 C1 Fagali'i 24900 Western 050 C1 Fagamalo 25700 Western 050 C1 Faganeanea 26500 Eastern 010 C1 Fagasa 27300 Eastern 010 C1 Fagatogo 28900 Eastern 010 C1 Failolo 29700 Western 050 C1 Faleasao 30500 Manu'a 020 C1 Faleniu 32100 Western 050 C1 Fatumafuti 32900 Eastern 010 C1 Futiga 36100 Western 050 C1 Ili'ili 36900 Western 050 C1 Lauli'i 40800 Eastern 010 C1 Leloaloa 44100 Eastern 010 C1 Leone 44900 Western 050 C1 Leusoali'i 45700 Manu'a 020 C1 Page 1 of 2 NCHS Instruction Manual Part 8A, Vital Records Geographic Classification, 2004 II. States, U. -

Geologic Map of National Park of American Samoa
National Park of American Samoa National Park Service American Samoa U.S. Department of the Interior Geologic Map of National Park of American Samoa Pt 25 25 20 2o5 $ o 20 o o o S# 20 $PL$UG 25 25 o $ Pt o$ o 5 $ Ra o 3 $ Pm o Ra $$ $ $ 2 o 10 Pt $ o $ $ Ra Pol P a c i f i c O c e a n 20$ 10 $ o o10 Ra Ra Ra o Pa 15 Ra $ $ Ra Ppe $ o o 25 Ra $ Pt $ $o $ 15 Ra o $ $ 18 o $ Ppt Ra 15 o3o5 10o 20 10 $ o $ 18 5o18 35 $ $ o 25 o Tutuila o o $ Ra o Ppt $ $ Ppi 15 10 $22o $ o $ 32 o $ Ra Rat 15 8 20 o Ra S# o 25 o$ $ 25 Ra 10 $ 2$0$ S#o$ o o 15 o Ra $$$6 $$ o $ Ra S# o 10 o o Pt o $ 35 $ $ Ppi 55 28 $ 6 $ o $ $ o Ppe $$ Ppt Ppi $ 5 Ra S# 10 Ra o 8 o $$$ $ o Ra Ra Po Ppeo Ra 15 16 $ 15 Qb o Qa 7o o14 o7 $ o oo Qa Rlc Qft 24 Qlel o o o 7 15 20 10 20 34 Tle Qfl $ o o $ $ o 25 Qlp Ra $$ 14 o 10 Ra o o $ 19 Rlc Landfill e e $ Qb Qtc 11 o $ Ofu o $ $ 14 17 Ra Ra Rll e Qte 1o1 Po 12 Qa o o Olosega 10 13 o Qa o o28 18 o e e 5 o 6 o e Qb 15 10 Qle Qnt Ttai Qa o 5 o 15 o Rla 22 13 24 Qb 6 Tle o o o o o Tafb o 15 Rll Qb o Qeli o Qb o e15 18 o 17 Ttae Tts e1o 0 10 Qa Qa Qlil e o Rlt 20 Qnl 6 11 o Qa Qa o o 6 15 o Qa 3 o 23 Qb Qa 10 o 2 o 35 o Tat 16 o 9 o Qb o Qb 10o 24 o 14 T'au o 7 o 19 10 o o P a c i f i c O c e a n 11o Qb e P a c i f i c O c e a n P a c i f i c O c e a n National Park of American Samoa Boundary Linear Dikes Geologic Units This map graphically presents digital geologic data prepared as part of the NPS Geologic Resources Qlp - Luatele Formation, pahoehoe flows Ppi - Pago Volcanic Series, lithic-vitric tuff Qnt - Nu’u Formation—tuff landfill Division’s Geologic Resource Evaluation Program. -

Download Map (PDF | 910.93
This map illustrates satellite-detected areas of likely building and vegetation damages caused by the magnitude 8.0 earthquake and resulting tsunami on 29 September 2009. Disaster coverage by the Earthquake 20 October 2009 A total of 11 damage sites were identified based on post-disaster satellite imagery International Charter 'Space and & Tsunami collected on 2-3 October 2009. Each site has a corresponding high resolution focus Major Disasters'. For more information on the Charter, which map within the PDF document. Damage identification was significantly restricted by is about assisting the disaster Version 1.0 heavy cloud and shadow coverage, as well as by spatial resolution of the imagery. It is relief organizations with multi- probable therefore that damages have been underestimated. This assessment is a satellite data and information, visit Glide No: preliminary analysis & has not yet been validated in the field. Please send ground www.disasterscharter.org !% !6 Damage Analysis with Formosat-2 Data Acquired on 2-3 October 2009 feedback to UNITAR / UNOSAT. !! TS-2009-000209-ASM 170°50'0"W 170°47'30"W 170°45'0"W 170°42'30"W 170°40'0"W 170°37'30"W 170°35'0"W NOTE: DAMAGE IDENTIFICATION WAS SIGNIFICANTLY RESTRICTED BY HEAVY CLOUDS AND SHADOW - SOUTHERN & EASTERN COASTS NOT ASSESSED Vatia Amalau Tafeu Bay Bay Cove Vatia 14°15'0"S Afona Masefau Tula 14°15'0"S Bay Bay Onenoa Pago Pago Masefau Port Sa'ilele Facility Afono Masausi Aoa Alao Leloaloa Fagaitua Pagai Anua Atuu Utulei Amaua Faga'itua D Pago Beach Aua Auasi Utumea T Bay TD Pago -

National Park Feasibility Study: American Samoa
NATIONAL PARK FEASIBILITY STUDY AMERICAN SAMOA July 1988 DRAFT Prepared by the National Park Service and the American Samoa Government TABLE OF CONTENTS Paae No. SUMMARY .............. BACKGROUND AND INTRODUCTION 3 Purpose ....... 3 Congressional Direction 3 The Study Area . 7 Previous Studies . 7 Consultation and Coordination 8 RESOURCES OF AMERICAN SAMOA I l Natural Resources . 11 Geology ........... l 1 Soils and Hydrology . 13 Coastal and Marine Resources . 14 Plant Life .... 15 Animal Life ... 17 Cultural Resources 28 Pre-history . 28 History...... 29 National Register of Historic Places 31 Legendary and Archeological Sites . 35 Scenic Resources . 39 PLANNING CONSIDERATIONS 41 Government ...... 41 Population and Economy 42 Tourism ... 45 Land Use .. 47 Land Tenure 54 SIGNIFICANCE, SUITABILITY, AND FEASIBILITY 57 Criteria for Park Lands ..... 57 Significant Areas and Sites Survey 58 Areas of National Significance 65 Suitability and Feasibility . 70 Management Alternatives . 77 POTENTIAL NATIONAL PARKS . 79 Description . 79 Potential National Park, Tutuila . 79 Potential National Park, Ta'u . 88 Concepts for Management, Development, and Visitor Use 97 Management Goals . 97 Development and Visitor Use, Tutuila . 99 Development and Visitor Use, Ta'u . 103 DRAFT 07/88 l Page No . PARK PROTECTION ALTERNATIVES . 108 ECONOMIC AND SOCIAL IMPACTS AND ENVIRONMENTAL CONSEQUENCES. 112 Environmental Consequences . 114 POSSIBLE ADDITIONS . 116 STUDY PARTICIPANTS . 119 BIBLIOGRAPHY . 121 APPENDICES. 125 Appendix 1. Summary of Village Meetings . 126 Appendix 2. Chronology of Archeological Survey Work . 131 Appendix 3. Potential Organization Chart of Fully Staffed National Parks. 133 Appendix 4. Summary of Public Meeting, Fono Guest House, Pago Pago. 135 DRAFT 07/88 ii LIST OF FIGURES Page No. Figure 1.