Recovery from Disturbance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

American Samoa
Coral Reef Habitat Assessment for U.S. Marine Protected Areas: U.S. Territory of American Samoa National Oceanic and Atmospheric Administration NOAA’s National Ocean Service Management & Budget Office Special Projects February 2009 Project Overview About this Effort NCCOS Benthic Habitat Mapping Effort The United States Coral Reef Task Force (USCRTF), in both its National The National Oceanic and Atmospheric Administration (NOAA) National Action Plan to Conserve Coral Reefs (2000) and its National Coral Reef Ocean Service (NOS) initiated a coral reef research program in 1999 to Action Strategy (2002), established a key conservation objective of pro- map, assess, inventory, and monitor U.S. coral reef ecosystems (Monaco tecting at least 20% of U.S. coral reefs and associated habitat types in et al. 2001). These activities were implemented in response to require- no-take marine reserves. NOAA’s Coral Reef Conservation Program has ments outlined in the Mapping Implementation Plan developed by the Map- been supporting efforts to assess current protection levels of coral reefs ping and Information Synthesis Working Group (MISWG) of the Coral Reef within Marine Protected Areas (MPAs) and quantify the area of U.S. coral Task Force (CRTF) (MISWG 1999). NOS’s National Centers for Coastal reef ecosystems protected in no-take reserves. The official federal defini- Ocean Science (NCCOS) Biogeography Team was charged with the de- tion of an MPA, signed into law by Executive Order 13158, is “any area of velopment and implementation of a plan to produce comprehensive digital the marine environment that has been reserved by federal, state, tribal, coral-reef ecosystem maps for all U.S. -

Warrior Nation Crushes Faga'itua's Hope of a Postseason
SECTION B VISIT SAMOA NEWS ONLINE @ SAMOANEWS.COM MONDAY, OCTOBER 16, 2017 CLASSIFIEDS • CARTOONS • ALOHA BRIEFS & MORE ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ ▼ C M Y K Warrior Nation crushes Faga’itua’s hope of a postseason TAFUNA REMAINS THE ONLY UNDEFEATED TEAM by T. Gasu a tough position. Samoa News Sports The Warriors claimed vic- Correspondent tory from the beginning of the ASHSAA’s sixth week of match, as they scored during high school football action at their fi rst offensive possession the Veterans Memorial Stadium of the game. this past weekend was also the After receiving the opening opening of the second round of kick off and returning it to the regular season for both var- midfi eld, the Warriors’ offen- O’Neil Ah Ching of Faga’itua returning a kick o all the way into Warriors territory – the longest sity and JV divisions. sive unit, led by Tala Sufi a, conversion for the Vikings during the match. Faga’itua went scoreless 0 to 24, in this JV match up. Many were hoping for a moved the chains downfi eld [photo: TG] Tafuna loss against the Vikings and spotted a fortunate fi rst last weekend, so the two pow- down situation on Faga’itua’s erhouses could see each other 15-yard line. An inside hand in post season playoffs but the off to Panapa Filoiali’i put the Warrior Nation said, “NOT Warriors on the board, with just THIS TIME,” as Tafuna sent 8:15 into the opening quarter both Vikings divisions home of the game. The two-point with losses. For now, Tafuna conversion pass was caught by remains the only undefeated Pene Fa’avae in the end zone team in the league, so their to put the Warriors on top with quest for a championship title an 8-point lead. -

CHAPTER II Planning Area Profile for Hazard Mitigation Analysis
CHAPTER II Planning Area Profile for Hazard Mitigation Analysis 24 Territory of American Samoa Multi-Hazard Mitigation Plan A U.S. Territory since 1900, American Samoa is located in the central South Pacific Ocean, 2,300 miles south-southwest of Hawaii and 1,600 miles east-northeast of New Zealand. American Samoa has a total land area of approximately 76 square miles and consists of a group of five volcanic islands and two atolls (Rose Atoll and Swains Island). The five volcanic islands, Tutuila, Aunu’u, Ofu, Olosega, and Ta’u, are the major inhabited islands. Tutuila is the largest island and the center of government. Ofu, Olosega, and Ta’u, collectively are referred to as the Manu’a Islands. Figure 1 Base Map of American Samoa depicts all of the islands of American Samoa. Figure 1. Base Map of American Samoa. The five volcanic islands, Tutuila, Aunu’u, Ofu, Olosega, and Ta’u, are the inhabited islands. At 53 square miles, Tutuila is the largest and oldest of the islands, and is the center of government and business. It is a long, narrow island lying SW-NE, is just over 20 miles in length, and ranges from 1 to 2 miles wide in the eastern half, and from 2 to 5 miles wide in the western half. Home to 95 percent of the territory’s 55,000 residents, Tutuila is the historic capitol (Pago Pago), the seat of American Samoa’s legislature and judiciary (Fagatogo), as well as the office of the Governor. Tutuila is often divided into 3 regions: the eastern district, the western district and Manu’a district. -

United States Department of the Interior Geological Survey the October 28, 1979, Landsliding on Tutuila, American Samoa by Jane
United States Department of the Interior Geological Survey The October 28, 1979, Landsliding On Tutuila, American Samoa by Jane M. Buchanan-Banks Open-File Report 81-81 This report is preliminary and has not been edited or reviewed for conformity with U. S. Geological Survey editorial standards and stratigraphic nomenclature The October 28, 1979, Landsliding on Tutuila, American Samoa Abstract In an apparently short time interval in the early morning of October 28, 1979, more than 70 landslides occurred on Tutuila Island, American Samoa. They were confined to the western end of the island from Asili on the south shore to Massacre Bay on the north. One of the landslides in Seetaga caused four fatalities; the others caused property damage and blocked the main transportation route on the west end of the island. Although there were rumors of an earthquake immediately preceding the landsliding, an examination of the seismograph from Apia Observatory, Western Samoa, for the morning of October 28 shows no record of an earth quake. Heavy rains during the preceding few days coupled with the general ly steep slopes and deeply weathered geologic materials appear to have caused the slope failures. There is abundant evidence both visually and on aerial photographs taken in 1941 and 1960 that landslides are frequent occurrences in the effected area. It is recommended that an analysis be made of the regional slope stability relative to current and proposed land use practices. Such an analysis would include: 1) air photos taken at a scale of 1:24,000 for comparison with those taken previously to document areas of landsliding; 2) a study of the vegetative cover; 3) a slope map; and 4) a detailed geological and structural map. -

“Food Desert” American Samoa Assessing Food Desert at School Locations
“Food Desert” American Samoa Assessing food desert at school locations. Molly Asifoa-Lagai Food Policy Analyst Community and Natural Resources Division American Samoa Community College July 2012 ACKNOWLEDGMENT Sincere gratitude is hereby extended to the following that assisted and helped with this report: Tapa’au Dr. Daniel Aga, ASCC-CNR Dean & Director Aufa’i Ropeti Apulu Areta, ASCC-CNR Associate Director/Extension Coordinator Dr. Don Vargo, ASCC-CNR Research Coordinator Emma Apatu, East Tennessee State Doctoral Student Kasie Richards, East Tennessee State Doctoral Student Neil Gurr, ASCC-CNR GIS Specialist Tipisone Fa’alogo, ASCC-CNR Media Specialist Rocky Mane, ASCC-CNR Media Technician Mary Scanlan, ASCC-CNR Research Assistant All ASCC-CNR Staff This work has been partially funded by Agricultural Development in the American Pacific project (ADAP)- NIFA Grant #2009-38826-19927. Financial support was also provided by a US Department of Agriculture Hatch grant, CRIS Accession No. 0212428, administered by the American Samoa Community College. 2 Executive Summary American Samoa is an unincorporated US territory in the South Pacific. It consists of the Tutuila and the Manua islands. The population is estimated at 70,000. This is an island nation that is currently having an increasing problem with obesity. The US had done studies, research and implemented programs to help prevent the increasing rate of obesity. The data from studies in the US have shown one in five children suffer from child obesity. It was estimated in 2009 that 55.6% of American Samoan schoolchildren were either overweight or obese, which is more than half of the children population in the territory. -

Summary of Ground-Water Data for Tutuila and Aunuu, American Samoa, for October 1987 Through September 1997
U.S. Department of the Interior Summary of Ground-Water Data for Tutuila and Aunuu, American Samoa, for October 1987 Through September 1997 U.S. GEOLOGICAL SURVEY Open-File Report 99-252 OCEAN Bay Prepared In cooperation with the AMERICAN SAMOA ENVIRONMENTAL PROTECTION AGENCY Summary of Ground-Water Data for Tutuila and Aunuu, American Samoa, for October 1987 Through September 1997 By Scot K. Izuka U.S. GEOLOGICAL SURVEY Open-File Report 99-252 Prepared in cooperation with the AMERICAN SAMOA ENVIRONMENTAL PROTECTION AGENCY Honolulu, Hawaii 1999 U.S. DEPARTMENT OF THE INTERIOR BRUCE BABBITT, Secretary U.S. GEOLOGICAL SURVEY Charles G. Groat, Director The use of firm, trade, and brand names in this report is for identification purposes only and does not constitute endorsement by the U.S. Geological Survey. For additional information write to: Copies of this report can be purchased from: District Chief U.S. Geological Survey U.S. Geological Survey Branch of Information Services 677 Ala Moana Blvd., Suite 415 Box 25286 Honolulu, HI 96813 Denver, CO 80225-0286 CONTENTS Abstract.......................................................................................... 1 Introduction....................................................................................... 1 Setting...................................................................................... 2 Acknowledgments ............................................................................ 2 Rainfall ......................................................................................... -

ALOFAU Village 14.263453S 170.619074W 170.591294W
14.263453S CENSUS 2000 BLOCK MAP: ALOFAU Village 14.263453S 170.619074W 170.591294W LEGEND SYMBOL NAME STYLE INTERNATIONAL AIR (FEDERAL) Trust Land / Home Land OTSA / TDSA / ANVSA AMERICAN INDIAN TRIBAL SUBDIVISION AIR (State) SDAISA Masefau ANRC 54500 Masausi 53700 MINOR CIVIL DIV. / CCD 1 Consolidated City Place within Subject Entity Incorporated Place / CDP 1 Place outside of Subject Entity Incorporated Place / CDP 1 Census Tract 2 ABBREVIATION REFERENCE: AIR = American Indian Reservation; Trust Land = Off−Reservation Trust Land; Home Land = Hawaiian Home Land; OTSA = Oklahoma Tribal Statistical Area; TDSA = Tribal Designated Statistical Area; ANVSA = Alaska Native Village Statistical Area; SDAISA = State Designated American Indian Statistical Area; ANRC = Alaska Native Regional Corporation; CDP = Census Designated Place; Minor Civil Div. (MCD) = Minor Civil Division; CCD = Census County Division FEATURES Sa'ilele 66500 Aoa 12100 VAIFANUA CO 86500 Military Inset Out Area Where international, state, and/or county boundaries coincide, the map shows the boundary symbol for only the highest−ranking of these boundaries. 1 A ' * ' following a place name indicates that the place is coextensive with a MCD, A ' ° ' indicates that the place is also a false MCD; the false MCD name is not shown. 2 A ' * ' following a block number indicates that the block number is repeated elsewhere in the block. SA'OLE CO 67300 Pagai 62230 SUA CO 72100 3007 Faga'itua 23300 3000 3006 Tutuila Island Fagaitua Bay 3005 Alofau SA'OLE CO 07300 67300 3002 3001 Amouli 10500 9502 3004 3003 Key to Adjacent Sheets 1 SHEET 1 OF 1 PARENT SHEET Amaua 09700 TOTAL SHEETS: 1 (Index 0; Parent 1; Inset 0) 14.290360S 14.290360S 170.619074W 170.591294W N ENTITY TYPE: Incorporated Place .2 .1 0 .2 mi W E NAME: Alofau village (07300) ST: American Samoa (60) S .3 .2 .1 0 .3 km CO: Eastern Dis (010) All legal boundaries and names are as of January 1, 2000. -

National List of Beaches 2004 (PDF)
National List of Beaches March 2004 U.S. Environmental Protection Agency Office of Water 1200 Pennsylvania Avenue, NW Washington DC 20460 EPA-823-R-04-004 i Contents Introduction ...................................................................................................................... 1 States Alabama ............................................................................................................... 3 Alaska................................................................................................................... 6 California .............................................................................................................. 9 Connecticut .......................................................................................................... 17 Delaware .............................................................................................................. 21 Florida .................................................................................................................. 22 Georgia................................................................................................................. 36 Hawaii................................................................................................................... 38 Illinois ................................................................................................................... 45 Indiana.................................................................................................................. 47 Louisiana -

Inventory and Monitoring of Seabirds in National Park of American Samoa
Final Report. Technical Report 136 INVENTORY AND MONITORING OF SEABIRDS IN NATIONAL PARK OF AMERICAN SAMOA By Paul J. O’Connor and Mark J. Rauzon October 2004 UNIVERSITY OF HAWAI’I AT MANOA NATIONAL PARK SERVICE CONTRACT NO. 8036-2-9004 CONTRIBUTION NUMBER 136 PSCU/UH - i - Item Page Table of Contents i List of Figures iii List of Tables iv Report Summary 1 Project Introduction 3 Background 3 Study Area 4 Study Species 11 Methods 13 General Methods 13 Complete Island Surveys 15 Fixed Location Counts 17 High-Elevation Ground & 19 Cliff Nesters Results & Discussion 22 General Results & Discussion 22 Complete Island Surveys 22 Fixed location Counts 35 Seabird Colonies 38 Coastal Breeders 38 High-Elevation Ground & Cliff 40 Nesters Species Present 40 Nests 43 Petrel Distributions Elsewhere 44 in American Samoa Petrel Specimens & Natural 45 History Rose Atoll Seabirds 49 Conclusions 50 Monitoring & Its Future at 50 NPSA Seabird Population Status 52 Field Access & Operations 54 Developing Additional 55 Survey Methods Invasive Species 56 - ii - Invertebrates 56 Vertebrates 56 Rodent Control 60 Human Impacts 63 Recommendations 64 Tutuila Unit 64 Manu’a Units 65 Acknowledgments 66 References 67 Appendices 71-140 Appendix A: Seabird Accounts for American Samoa 71 Appendix B: Tutuila and Aunu’u Islands, Round Island 98 Survey Site Maps Appendix C: Tahiti Petrel Voice Analysis 112 Appendix D: Ectoparasites from Tahiti Petrel on Ta’u 116 Appendix E: Radar Survey Techniques Employed at 119 Channel Islands National Park Appendix F: Establishing a Water Collection System 123 and Base Camp on Mt. Lata Appendix G: Status of the Spotless Crake in American 124 Samoa Appendix H: Coastal Seabird Colonies Maps for 130 American Samoa Appendix I: Brief Video Clips from Summit of Mt. -
American Samoa State Code: AS
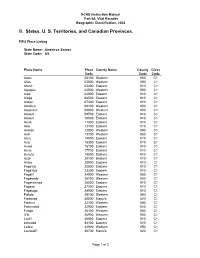
NCHS Instruction Manual Part 8A, Vital Records Geographic Classification, 2004 II. States, U. S. Territories, and Canadian Provinces. FIPS Place Listing State Name: American Samoa State Code: AS Place Name Place County Name County Class Code Code Code Aasu 00100 Western 050 C1 Afao 02500 Western 050 C1 Afono 03300 Eastern 010 C1 Agugulu 04500 Western 050 C1 Alao 04900 Eastern 010 C1 Alega 06500 Eastern 010 C1 Alofau 07300 Eastern 010 C1 Amaluia 08100 Western 050 C1 Amanave 08900 Western 050 C1 Amaua 09700 Eastern 010 C1 Amouli 10500 Eastern 010 C1 Anua 11300 Eastern 010 C1 Aoa 12100 Eastern 010 C1 Aoloau 12800 Western 050 C1 Asili 13700 Western 050 C1 Atu'u 14500 Eastern 010 C1 Aua 15300 Eastern 010 C1 Auasi 16100 Eastern 010 C1 Aumi 17700 Eastern 010 C1 Aunu'u 18500 Eastern 010 C1 Auto 20100 Eastern 010 C1 Avaio 20900 Eastern 010 C1 Faga'alu 22500 Eastern 010 C1 Faga'itua 23300 Eastern 010 C1 Fagali'i 24900 Western 050 C1 Fagamalo 25700 Western 050 C1 Faganeanea 26500 Eastern 010 C1 Fagasa 27300 Eastern 010 C1 Fagatogo 28900 Eastern 010 C1 Failolo 29700 Western 050 C1 Faleasao 30500 Manu'a 020 C1 Faleniu 32100 Western 050 C1 Fatumafuti 32900 Eastern 010 C1 Futiga 36100 Western 050 C1 Ili'ili 36900 Western 050 C1 Lauli'i 40800 Eastern 010 C1 Leloaloa 44100 Eastern 010 C1 Leone 44900 Western 050 C1 Leusoali'i 45700 Manu'a 020 C1 Page 1 of 2 NCHS Instruction Manual Part 8A, Vital Records Geographic Classification, 2004 II. States, U. -

Geologic Map of National Park of American Samoa
National Park of American Samoa National Park Service American Samoa U.S. Department of the Interior Geologic Map of National Park of American Samoa Pt 25 25 20 2o5 $ o 20 o o o S# 20 $PL$UG 25 25 o $ Pt o$ o 5 $ Ra o 3 $ Pm o Ra $$ $ $ 2 o 10 Pt $ o $ $ Ra Pol P a c i f i c O c e a n 20$ 10 $ o o10 Ra Ra Ra o Pa 15 Ra $ $ Ra Ppe $ o o 25 Ra $ Pt $ $o $ 15 Ra o $ $ 18 o $ Ppt Ra 15 o3o5 10o 20 10 $ o $ 18 5o18 35 $ $ o 25 o Tutuila o o $ Ra o Ppt $ $ Ppi 15 10 $22o $ o $ 32 o $ Ra Rat 15 8 20 o Ra S# o 25 o$ $ 25 Ra 10 $ 2$0$ S#o$ o o 15 o Ra $$$6 $$ o $ Ra S# o 10 o o Pt o $ 35 $ $ Ppi 55 28 $ 6 $ o $ $ o Ppe $$ Ppt Ppi $ 5 Ra S# 10 Ra o 8 o $$$ $ o Ra Ra Po Ppeo Ra 15 16 $ 15 Qb o Qa 7o o14 o7 $ o oo Qa Rlc Qft 24 Qlel o o o 7 15 20 10 20 34 Tle Qfl $ o o $ $ o 25 Qlp Ra $$ 14 o 10 Ra o o $ 19 Rlc Landfill e e $ Qb Qtc 11 o $ Ofu o $ $ 14 17 Ra Ra Rll e Qte 1o1 Po 12 Qa o o Olosega 10 13 o Qa o o28 18 o e e 5 o 6 o e Qb 15 10 Qle Qnt Ttai Qa o 5 o 15 o Rla 22 13 24 Qb 6 Tle o o o o o Tafb o 15 Rll Qb o Qeli o Qb o e15 18 o 17 Ttae Tts e1o 0 10 Qa Qa Qlil e o Rlt 20 Qnl 6 11 o Qa Qa o o 6 15 o Qa 3 o 23 Qb Qa 10 o 2 o 35 o Tat 16 o 9 o Qb o Qb 10o 24 o 14 T'au o 7 o 19 10 o o P a c i f i c O c e a n 11o Qb e P a c i f i c O c e a n P a c i f i c O c e a n National Park of American Samoa Boundary Linear Dikes Geologic Units This map graphically presents digital geologic data prepared as part of the NPS Geologic Resources Qlp - Luatele Formation, pahoehoe flows Ppi - Pago Volcanic Series, lithic-vitric tuff Qnt - Nu’u Formation—tuff landfill Division’s Geologic Resource Evaluation Program. -

Download Map (PDF | 910.93
This map illustrates satellite-detected areas of likely building and vegetation damages caused by the magnitude 8.0 earthquake and resulting tsunami on 29 September 2009. Disaster coverage by the Earthquake 20 October 2009 A total of 11 damage sites were identified based on post-disaster satellite imagery International Charter 'Space and & Tsunami collected on 2-3 October 2009. Each site has a corresponding high resolution focus Major Disasters'. For more information on the Charter, which map within the PDF document. Damage identification was significantly restricted by is about assisting the disaster Version 1.0 heavy cloud and shadow coverage, as well as by spatial resolution of the imagery. It is relief organizations with multi- probable therefore that damages have been underestimated. This assessment is a satellite data and information, visit Glide No: preliminary analysis & has not yet been validated in the field. Please send ground www.disasterscharter.org !% !6 Damage Analysis with Formosat-2 Data Acquired on 2-3 October 2009 feedback to UNITAR / UNOSAT. !! TS-2009-000209-ASM 170°50'0"W 170°47'30"W 170°45'0"W 170°42'30"W 170°40'0"W 170°37'30"W 170°35'0"W NOTE: DAMAGE IDENTIFICATION WAS SIGNIFICANTLY RESTRICTED BY HEAVY CLOUDS AND SHADOW - SOUTHERN & EASTERN COASTS NOT ASSESSED Vatia Amalau Tafeu Bay Bay Cove Vatia 14°15'0"S Afona Masefau Tula 14°15'0"S Bay Bay Onenoa Pago Pago Masefau Port Sa'ilele Facility Afono Masausi Aoa Alao Leloaloa Fagaitua Pagai Anua Atuu Utulei Amaua Faga'itua D Pago Beach Aua Auasi Utumea T Bay TD Pago