Basic Laboratory Tests Basic Blood Chemistry Tests
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CANINE INSULINOMA: DIAGNOSIS, TREATMENT, & STAGING Eliza Reiss Grant, DVM, and Kristine E
Peer Reviewed PRACTICAL ONCOLOGY CANINE INSULINOMA: DIAGNOSIS, TREATMENT, & STAGING Eliza Reiss Grant, DVM, and Kristine E. Burgess, DVM, Diplomate ACVIM (Oncology) Tufts University An insulinoma is a malignant pancreatic tumor that DIAGNOSIS inappropriately secretes excessive insulin, resulting in Aside from a histologic confirmation of insulinoma, profound hypoglycemia.1 no currently available diagnostic test provides a de- Pancreatic tumors are classified as: finitive diagnosis of insulinoma. Existing techniques • Exocrine, which includes adenocarcinomas of may help increase suspicion for an insulin-secreting ductular or acinar origin tumor but, with most diagnostic testing, it is im- • Endocrine, which arise from the islets of perative to interpret all results in the context of the Langerhans. coexisting clinical signs. Insulinomas are functional neuroendocrine tumors that originate in the beta cells of the islets Differential Diagnosis of Langerhans.1 A complete work-up, including careful patient history, physical examination, bloodwork, and PRESENTATION diagnostic imaging tests, should be performed to Signalment rule out other causes of hypoglycemia, such as Any breed of dog can be affected, but large sepsis, hepatic failure, adrenal cortical insufficiency, breeds tend to be overrepresented.1 While, in toxin ingestion, and other forms of neoplasia. humans, insulinomas affect females far more frequently than males, there is no apparent sex Laboratory Tests predilection in dogs.1-3 Dogs also commonly Blood Glucose present with a malignant variant, while humans A simple fasting blood glucose level of less than often have a benign adenoma (80%).1 Insulino- 40 mg/dL can suggest hyperinsulinemia, although ma is rare in cats.4 careful monitoring of a fasted dog with suspected insulinoma is strongly recommended due to high Clinical Signs risk for seizure activity. -

Metabolic Regulation of Heme Catabolism and Bilirubin Production
Metabolic Regulation of Heme Catabolism and Bilirubin Production. I. HORMONAL CONTROL OF HEPATIC HEME OXYGENASE ACTIVITY Arne F. Bakken, … , M. Michael Thaler, Rudi Schmid J Clin Invest. 1972;51(3):530-536. https://doi.org/10.1172/JCI106841. Research Article Heme oxygenase (HO), the enzyme system catalyzing the conversion of heme to bilirubin, was studied in the liver and spleen of fed, fasted, and refed rats. Fasting up to 72 hr resulted in a threefold increase in hepatic HO activity, while starvation beyond this period led to a gradual decline in enzyme activity. Refeeding of rats fasted for 48 hr depressed hepatic HO activity to basal values within 24 hr. Splenic HO was unaffected by fasting and refeeding. Hypoglycemia induced by injections of insulin or mannose was a powerful stimulator of hepatic HO. Glucose given together with the insulin abolished the stimulatory effect of the latter. Parenteral treatment with glucagon led to a twofold, and with epinephrine to a fivefold, increase of hepatic HO activity; arginine, which releases endogenous glucagon, stimulated the enzyme fivefold. These stimulatory effects of glucagon and epinephrine could be duplicated by administration of cyclic adenosine monophosphate (AMP), while thyroxine and hydroxortisone were ineffective. Nicotinic acid, which inhibits lipolysis, failed to modify the stimulatory effect of epinephrine. None of these hormones altered HO activity in the spleen. These findings demonstrate that the enzymatic mechanism involved in the formation of bilirubin from heme in the liver is stimulated by fasting, hypoglycemia, epinephrine, glucagon, and cyclic AMP. They further suggest that the enzyme stimulation produced by fasting may be […] Find the latest version: https://jci.me/106841/pdf Metabolic Regulation of Heme Catabolism and Bilirubin Production I. -

Jaundice Protocol
fighting childhood liver disease Jaundice Protocol Early identification and referral of liver disease in infants fighting childhood liver disease 36 Great Charles Street Birmingham B3 3JY Telephone: 0121 212 3839 yellowalert.org childliverdisease.org [email protected] Registered charity number 1067331 (England & Wales); SC044387 (Scotland) The following organisations endorse the Yellow Alert Campaign and are listed in alphabetical order. 23957 CLDF Jaundice Protocol.indd 1 03/08/2015 18:25:24 23957 3 August 2015 6:25 PM Proof 1 1 INTRODUCTION This protocol forms part of Children’s Liver Disease Foundation’s (CLDF) Yellow Alert Campaign and is written to provide general guidelines on the early identification of liver disease in infants and their referral, where appropriate. Materials available in CLDF’s Yellow Alert Campaign CLDF provides the following materials as part of this campaign: • Yellow Alert Jaundice Protocol for community healthcare professionals • Yellow Alert stool colour book mark for quick and easy reference • Parents’ leaflet entitled “Jaundice in the new born baby”. CLDF can provide multiple copies to accompany an antenatal programme or for display in clinics • Yellow Alert poster highlighting the Yellow Alert message and also showing the stool chart 2 GENERAL AWARENESS AND TRAINING The National Institute of Health and Clinical Excellence (NICE) has published a clinical guideline on neonatal jaundice which provides guidance on the recognition, assessment and treatment of neonatal jaundice in babies from birth to 28 days. Neonatal Jaundice Clinical Guideline guidance.nice.org.uk cg98 For more information go to nice.org.uk/cg98 • Jaundice Community healthcare professionals should be aware that there are many causes for jaundice in infants and know how to tell them apart: • Physiological jaundice • Breast milk jaundice • Jaundice caused by liver disease • Jaundice from other causes, e.g. -

Journal of Blood Group Serology and Molecular Genetics Volume 34, Number 1, 2018 CONTENTS
Journal of Blood Group Serology and Molecular Genetics VOLUME 34, N UMBER 1, 2018 This issue of Immunohematology is supported by a contribution from Grifols Diagnostics Solutions, Inc. Dedicated to advancement and education in molecular and serologic immunohematology Immunohematology Journal of Blood Group Serology and Molecular Genetics Volume 34, Number 1, 2018 CONTENTS S EROLOGIC M ETHOD R EVIEW 1 Warm autoadsorption using ZZAP F.M. Tsimba-Chitsva, A. Caballero, and B. Svatora R EVIEW 4 Proceedings from the International Society of Blood Transfusion Working Party on Immunohaematology Workshop on the Clinical Significance of Red Blood Cell Alloantibodies, Friday, September 2, 2016, Dubai A brief overview of clinical significance of blood group antibodies M.J. Gandhi, D.M. Strong, B.I. Whitaker, and E. Petrisli C A S E R EPORT 7 Management of pregnancy sensitized with anti-Inb with monocyte monolayer assay and maternal blood donation R. Shree, K.K. Ma, L.S. Er and M. Delaney R EVIEW 11 Proceedings from the International Society of Blood Transfusion Working Party on Immunohaematology Workshop on the Clinical Significance of Red Blood Cell Alloantibodies, Friday, September 2, 2016, Dubai A review of in vitro methods to predict the clinical significance of red blood cell alloantibodies S.J. Nance S EROLOGIC M ETHOD R EVIEW 16 Recovery of autologous sickle cells by hypotonic wash E. Wilson, K. Kezeor, and M. Crosby TO THE E DITOR 19 The devil is in the details: retention of recipient group A type 5 years after a successful allogeneic bone marrow transplant from a group O donor L.L.W. -

Comparative Evaluation of Fructosamine and Hba1c As a Marker of Glycemic Control in Type 2 Diabetes: a Hospital Based Study
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article Comparative Evaluation of Fructosamine and HbA1c as a Marker of Glycemic Control in Type 2 Diabetes: A Hospital Based Study Dr. Jyoti Goyal1, Dr. Nibhriti Das2, Dr. Navin Kumar3, Ms. Seema Raghav4, Dr. Paramjeet Singh Bhatia5, Dr. Karunesh Prasad Singh6, Dr. Sabari Das7 1DNB, Department of Internal Medicine, Nayati Healthcare and Research Centre, Mathura, India- 281003, 2Ex-Director of Laboratory services and Additional Dean Research and Academics, Nayati Healthcare and Research Centre, Mathura, India- 281003, 3Ph.D, Biostatistitian, Department of Biostatistics, Nayati Healthcare and Research Centre, Mathura, India-281003. 4M.Sc., Certified Diabetes Educator, Department of Internal Medicine, Nayati Healthcare and Research Centre, Mathura, India-281003. 5MD, Department of Internal Medicine, Nayati Healthcare and Research Centre, Mathura, India-281003. 6MD Physician, Department of Internal Medicine, Nayati Healthcare and Research Centre, Mathura, India-281003. 7Department of Laboratory Medicine, Nayati Healthcare and Research Centre, Mathura, India- 281003, Corresponding Author: Dr. Jyoti Goyal ABSTRACT Introduction: Management of type 2 diabetes revolves around achievement of target glycemic control with the help of antidiabetic drugs or insulin. There are various markers for measurement of glyceamic control like HbA1c, Mean Blood Glucose and fructosamine levels. Though HbA1c is a well validated standard method for assessment of glycemic control but it has also got certain limitations. Fructosamine, a less explored method may be used as an alternative marker for an assessment of glycemic control in cases where HbA1c is unreliable or unavailable. The objective of this study is to compare the fructosamine levels with HbA1c in assessment of glycemic control in type 2 diabetics so as to assess the utility of fructosamine as an alternative marker for evaluation of glucose control. -

Comparison of Histopathology, Immunofluorescence, and Serology
Global Dermatology Cae Report ISSN: 2056-7863 Comparison of histopathology, immunofluorescence, and serology for the diagnosis of autoimmune bullous disorders: an update Seline Ali E1, Seline Lauren N1, Sokumbi Olayemi1* and Motaparthi Kiran2,3 1Department of Dermatology, Medical College of Wisconsin, Milwaukee, WI, USA 2Dermatopathology, Miraca Life Sciences, USA 3Department of Dermatology, University of Texas Southwestern Medical Center, Dallas, TX, USA Introduction In an ELISA, the target antigen of interest (such as the NC16a domain of BP180) is immobilized by physical adsorption or by The diagnosis of autoimmune bullous disorders (AIBDs) relies on antibody capture. When antibody capture is utilized, this is referred to several different diagnostic methods. These include histopathology, as “sandwich ELISA” because the target antigen is bound between the direct immunofluorescence (DIF), indirect immunofluorescence immobilizing antibody and the primary antibody. Primary antibodies (IIF), enzyme-linked immunosorbent assay (ELISA) and are present in the patient’s serum. Enzyme-linked secondary antibodies immunoblotting. When faced with a presumptive AIBD, the are then added which bind the Fc region of primary antibodies. most widely employed method for diagnosis by dermatologists is Substrate is added and converted by the enzyme into a signal. A a combination of histopathology and DIF. While DIF is still the resulting color change, fluorescence, or electrochemical signal is diagnostic method of choice for linear IgA bullous disease and IgA quantitatively measured and reported [5]. pemphigus, ELISA is a more accurate, cost-effective and less invasive method of diagnosis for several AIBDs including pemphigus vulgaris Western blot is synonymous with immunoblot. For this method, and foliaceus, based on currently available evidence [1-3]. -

Serum Levels of True Insulin, C-Peptide and Proinsulin in Peripheral Blood of Patients with Cirrhosis
Diabetologia (1983) 25: 506-509 Diabetologia Springer-Verlag 1983 Serum Levels of True Insulin, C-Peptide and Proinsulin in Peripheral Blood of Patients with Cirrhosis T. Kasperska-Czy~ykowa 1, L. G. Heding 2 and A. Czy2yk 1 1Department of Gastroenterology and Metabolic Diseases, Medical Academy of Warsaw, Poland, and 2Novo Research Institute, Bagsvaerd, Denmark Summary. The levels of proinsulin, immunoreactive insulin, but the difference was less pronounced and only significant at true insulin (calculated from the difference, namely immuno- a few of the time points. The serum level of C-peptide was reactive insulin-proinsulin) and C-peptide were determined in very similar in both groups. These results emphasize that cir- the fasting state and during a 3-h oral glucose tolerance test af- rhosis is a condition in which the serum proinsulin level is ter administration of 100 g of glucose in 12 patients with cir- raised and that this hyperproinsulinaemia contributes greatly rhosis with normal oral glucose tolerance test (50 g) and in to the increased immunoreactive insulin levels observed in 12 healthy subjects serving as controls. In the patients with cir- patients with this disease. rhosis the serum levels of proinsulin and immunoreactive in- sulin were significantly higher in the fasting state and after Key words: Insulin, cirrhosis, C-peptide, proinsulin, oral glu- glucose loading than in the healthy subjects. The serum level cose tolerance test. of true insulin was also higher in the patients with cirrhosis, After the introduction of a radioimmunoassay for se- Patients and Methods rum (plasma) insulin determination (IRI) many authors reported raised levels of this hormone in the peripheral blood of patients with cirrhosis [1, 5-7, 9, 16-19]. -

Online-Only Appendix: List of Inclusion and Exclusion Criteria
ONLINE-ONLY APPENDIX: LIST OF INCLUSION AND EXCLUSION CRITERIA INCLUSION CRITERIA [1] Male subjects between the ages of 35 and 70 years, inclusive, with Type 2 diabetes mellitus as defined by the American Diabetes Association (Diabetes care, Vol. 21: S5- S19, 1998) for more than one year. [2] Subjects must have Body Mass Index (BMI) < 36 kg/m². [3] Stable glycemic control (Hb A1C <11%). [4] Subjects must be off all oral hypoglycemic agents 24 hours prior to each study dosing day and off any investigational drug for at least four weeks. [5] Subjects must refrain from strenuous physical activity beginning 72 hours prior to admission and through the duration of the study. [6] Subjects must be willing and able to be confined to the Clinical Research Unit as required by the protocol. [7] Subjects must be willing and able to provide written informed consent. EXCLUSION CRITERIA [1] History or presence of clinically significant cardiovascular, respiratory, hepatic, renal, gastrointestinal, neurological or infectious disorders capable of altering the absorption, metabolism or elimination of drugs, or of constituting a risk factor when taking the study medication. [2] Subjects with gastroparesis, orthostatic hypotension and hypoglycemia unawareness (autonomic neuropathy). [3] Subjects with “brittle” diabetes or predisposition to severe hypoglycemia, e.g., 2 or more serious hypoglycemic episodes (requiring another’s assistance) within the past year, or any hospitalization or emergency room visit due to poor diabetic control within the past 6 months. [4] Evidence of significant active hematological disease and/or cumulative blood donation of 1 unit (500 mL) or more including blood drawn during clinical trials in the last 3 months. -

A Novel Perspective on the Biology of Bilirubin in Health and Disease
Opinion A Novel Perspective on the Biology of Bilirubin in Health and Disease 1,z 2,z, 3 Silvia Gazzin, Libor Vitek, * Jon Watchko, 4,5,6,7 1,8, Steven M. Shapiro, and Claudio Tiribelli * Unconjugated bilirubin (UCB) is known to be one of the most potent endogenous Trends antioxidant substances. While hyperbilirubinemia has long been recognized as Historically known for its toxicity but an ominous sign of liver dysfunction, recent data strongly indicate that mildly recently recognized as a powerful pro- tective molecule, BLB is gaining more elevated bilirubin (BLB) levels can be protective against an array of diseases attention due to its pleiotropic biomo- associated with increased oxidative stress. These clinical observations are lecular effects and those of the supported by new discoveries relating to the role of BLB in immunosuppression enzymes involved in BLB metabolism (the ‘Yellow Players’). and inhibition of protein phosphorylation, resulting in the modulation of intra- cellular signaling pathways in vascular biology and cancer, among others. Both heme oxygenase (HMOX) and bili- Collectively, the evidence suggests that targeting BLB metabolism could be verdin reductase (BLVR) (the main enzymes in BLB metabolism) act on considered a potential therapeutic approach to ameliorate a variety of numerous signaling pathways, with conditions. unsuspected biological consequences. The interconnections of such pathways highlight an incredibly complex biomo- From a Biological Waste Product to a Potent Biological Compound lecular network. Yellow player mole- UCB (see Glossary), the end product of the heme catabolic pathway, has long been recognized cules can have important physiological as a sign of liver dysfunction or a potential toxic factor causing severe brain damage in newborns. -

Application of Quantitative Immunofluorescence to Clinical Serology: Antibody Levels of Treponema Pallidum GRACE L
JOURNAL OF CLINICAL MICROBIOLOGY, May 1992, p. 1294-1296 Vol. 30, No. 5 0095-1137/92/051294-03$02.00/0 Copyright C 1992, American Society for Microbiology Application of Quantitative Immunofluorescence to Clinical Serology: Antibody Levels of Treponema pallidum GRACE L. PICCIOLO* AND DAVID S. KAPLAN Center for Devices and Radiological Health, Food and Drug Administration, 12200 Wilkins Avenue, Rockville, Maryland 20852 Received 27 March 1991/Accepted 20 January 1992 A previously reported method of quantitative immunofluorescence, employing a calibrated photometric system and chemically stabilized fluorescence intensity, was used to replace the subjective, visual method of endpoint determination with a quantitative, calibrated measurement of antibodies to Treponema paUlidum in serum. The results of the quantitative immunofluorescence method showed a 90% correlation with the subjective determinations of the visual method. A quantitative immunofluorescence (QIF) method to de- measured by using an acridine orange filter set, with a 400- to termine immunofluorescence with a quantitative, calibrated 440-nm excitation and LP 470 emission filters. photometric intensity of the fluorescent reaction product has Reducing agent. Dithioerythritol (DTE) (Sigma Chemical been reported previously by us (4). This method used uranyl Co., St. Louis, Mo.) was prepared as stock solution contain- glass slides in the calibration and standardization of the ing 0.3 M reducing agent in 0.5 M Tris buffer, pH 8.2 (Sigma microscope-photometer voltage measurements. To stabilize Chemical Co.). DTE was diluted 1:9 with standard buffered the fluorescence emission, dithioerythritol (DTE) was incor- glycerol mounting medium (Clinical Sciences, Inc., Whip- porated into the buffered glycerol mounting medium of the pany, N.J.) (4, 8). -
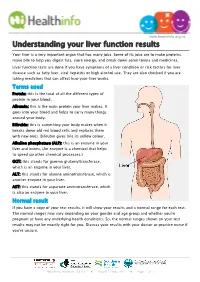
Understanding Your Liver Function Results
www.healthinfo.org.nz Understanding your liver function results Your liver is a very important organ that has many jobs. Some of its jobs are to make proteins, make bile to help you digest fats, store energy, and break down some toxins and medicines. Liver function tests are done if you have symptoms of a liver condition or risk factors for liver disease such as fatty liver, viral hepatitis or high alcohol use. They are also checked if you are taking medicines that can affect how your liver works. Terms used Protein: this is the total of all the different types of protein in your blood. Albumin: this is the main protein your liver makes. It goes into your blood and helps to carry many things around your body. Bilirubin: this is something your body makes when it breaks down old red blood cells and replaces them with new ones. Bilirubin gives bile its yellow colour. Alkaline phosphatase (ALP): this is an enzyme in your liver and bones. (An enzyme is a chemical that helps to speed up other chemical processes.) GGT: this stands for gamma glutamyltransferase, which is an enzyme in your liver. ALT: this stands for alanine aminotransferase, which is another enzyme in your liver. AST: this stands for aspartate aminotransferase, which is also an enzyme in your liver. Normal result If you have a copy of your test results, it will show your results and a normal range for each test. The normal ranges may vary depending on your gender and age group and whether you're pregnant or have any underlying health conditions. -

Clinical Placements Serology Screening for Students
Gawler Place Medical Practice Level 1, Key Invest Building 49 Gawler Place Adelaide SA 5000 T: 08 8212 7175 F: 08 8212 1993 E: [email protected] www.adelaideunicare.com.au CLINICAL PLACEMENTS SEROLOGY SCREENING FOR STUDENTS Gawler Place Medical Practice have put together this Question and Answer sheet to assist you in meeting the requirements of SA Health’s Immunisation for Health Care Workers in South Australia Policy – August 2017. It is a requirement that all Health Care Workers have adequate immunisation against certain diseases prior to working in a clinical environment. What is Serology Screening and why do I need to do it? Serology Screening is a blood test that looks for antibodies in your blood. The purpose of such a test is to detect serum antibodies to prove protective immunity against a disease. Where can I have Serology Screening done? Gawler Place Medical Practice offer Serology Screening appointments to allow incoming students to have their serology screening completed, and where required, obtain their required vaccinations to meet SA Health’s Immunisation for Health Care Workers in South Australia – August 2017. Australian Clinical Labs are located within our Practice so that you can have your blood taken (if required) as soon as you have seen the doctor. How do I make an appointment? You may contact the practice to organise an appointment, our telephone number is 8212 7175 or make a booking online. Please make a long appointment booking. If you need to change or cancel an appointment please contact our Practice on (08) 2127175 as soon as possible so that your appointment can be offered to another person.