Jaundice Protocol
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Understanding Your A1C Test
Diabetes Advisor Understanding Your A1C Test What is the A1C test? The A1C is a blood test that tells you what your average blood sugar (blood glucose) levels have been for the past two to three months. It measures how much sugar is attached to your red blood cells. If your blood sugar is frequently high, more will be attached to your blood cells. Because you are always making new red blood cells to replace old ones, your A1C changes over time as your blood sugar levels change. “Because you are always making new What is eAG? red blood cells to eAG stands for estimated average glucose and is your estimated average blood replace old ones, sugar. This number translates an A1C test result into a number like the one you see when you test your blood sugar at home. For example, an A1C of 7% means your A1C changes that your average sugar for the last two to three months was about 154 mg/dL. over time as your What does an A1C/eAG result mean? blood sugar levels change.” Usually, your A1C gives you general trend in your blood sugar that matches what you see with your day-to-day blood sugar checks. Sometimes, however, your A1C result may seem higher or lower than you expected. That may be because you aren’t checking your blood sugar at times when it’s very high or very low. Use the chart below to understand how your A1C result translates to eAG. First find your A1C number on the left. -

Serological (Antibody) Testing for Covid-19
SEROLOGICAL (ANTIBODY) TESTING FOR COVID-19 Serological tests detect antibodies in the blood People in the early stages of COVID-19 might generated as part of the immune response test antibody negative despite being highly to a specific infection, such as infection with infectious. Additionally, some tests might give a SARS-CoV-2, the virus that causes COVID-19. false positive result because of past or present Antibody tests are different from tests such as infection with other types of coronaviruses. polymerase chain reaction (PCR) and antigen False positive results are also more likely tests which detect the SARS-CoV-2 virus. when the percentage of the population with Many new serological tests for COVID-19 have the disease is low. The Idaho Division of Public been developed and have an emergency use Health discourages persons who have a positive authorization (EUA) from the U.S. Food and serology test from relaxing the precautions such Drug Administration (FDA). Only antibody tests as social distancing that are recommended for that have an FDA EUA should be used. The all Idahoans to prevent spread of coronavirus, Idaho Division of Public Health discourages and strongly discourages employers form the use of unauthorized serology-based assays relaxing the employee protections for an for diagnosis of COVID-19 or determining if employee solely based upon a positive serology someone is currently infected or had a prior test. infection. The immune response to SARS-CoV-2 (the Serological tests are not recommended for virus that causes COVID-19) infection is not COVID-19 diagnosis in most situations because well understood. -

Metabolic Regulation of Heme Catabolism and Bilirubin Production
Metabolic Regulation of Heme Catabolism and Bilirubin Production. I. HORMONAL CONTROL OF HEPATIC HEME OXYGENASE ACTIVITY Arne F. Bakken, … , M. Michael Thaler, Rudi Schmid J Clin Invest. 1972;51(3):530-536. https://doi.org/10.1172/JCI106841. Research Article Heme oxygenase (HO), the enzyme system catalyzing the conversion of heme to bilirubin, was studied in the liver and spleen of fed, fasted, and refed rats. Fasting up to 72 hr resulted in a threefold increase in hepatic HO activity, while starvation beyond this period led to a gradual decline in enzyme activity. Refeeding of rats fasted for 48 hr depressed hepatic HO activity to basal values within 24 hr. Splenic HO was unaffected by fasting and refeeding. Hypoglycemia induced by injections of insulin or mannose was a powerful stimulator of hepatic HO. Glucose given together with the insulin abolished the stimulatory effect of the latter. Parenteral treatment with glucagon led to a twofold, and with epinephrine to a fivefold, increase of hepatic HO activity; arginine, which releases endogenous glucagon, stimulated the enzyme fivefold. These stimulatory effects of glucagon and epinephrine could be duplicated by administration of cyclic adenosine monophosphate (AMP), while thyroxine and hydroxortisone were ineffective. Nicotinic acid, which inhibits lipolysis, failed to modify the stimulatory effect of epinephrine. None of these hormones altered HO activity in the spleen. These findings demonstrate that the enzymatic mechanism involved in the formation of bilirubin from heme in the liver is stimulated by fasting, hypoglycemia, epinephrine, glucagon, and cyclic AMP. They further suggest that the enzyme stimulation produced by fasting may be […] Find the latest version: https://jci.me/106841/pdf Metabolic Regulation of Heme Catabolism and Bilirubin Production I. -

Lactose Tolerance Blood Test
Lactose tolerance blood test Lactose tolerance tests measure the ability of your intestines to break down lactose, a type of sugar found in milk and other dairy products. How the test is performed The lactose tolerance blood test looks for glucose in your blood. Your body creates glucose when lactose breaks down. For this test, several blood samples will be taken before and after you drink the lactose solution described above. For information on how a blood sample is obtained, see venipuncture. How to prepare for the test You should not eat for 8 hours before the test. Avoid strenuous exercise for 8 hours before the test. How the test will feel There should not be any pain or discomfort when giving a breath sample. When the needle is inserted to draw blood, some people feel moderate pain, while others feel only a prick or stinging sensation. Afterward, there may be some throbbing. Why the test is performed Your doctor may order these tests if you have signs of lactose intolerance. Normal Values The breath test is considered normal if the increase in hydrogen is less than 12 parts per million over your fasting (pre-test) level. The blood test is considered normal if your glucose level rises more than 30 mg/dL within 2 hours of drinking the lactose solution. A rise of 20-30 mg/dL is inconclusive. Note: Normal value ranges may vary slightly among different laboratories. Talk to your doctor about the meaning of your specific test results. The examples above show the common measurements for results for these tests. -

Rotational Thromboelastometry Predicts Care Level in Covid-19
medRxiv preprint doi: https://doi.org/10.1101/2020.06.11.20128710; this version posted June 12, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY-NC-ND 4.0 International license . Rotational Thromboelastometry predicts care level in Covid-19 1 2 3 4 Lou M. Almskog, MD ; Agneta Wikman, MD, PhD ; Jonas Svensson, MD ; 1 5 6 2 Michael Wanecek, MD, PhD ; Matteo Bottai, PhD ; Jan van der Linden, MD, PhD 7 8 9 ; Anna Ågren, MD, PhD . 1 Department of Anaesthesiology and Intensive Care, Capio St Göran’s Hospital, Stockholm, Sweden 2 Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden 3 Department of Clinical Immunology and Transfusion Medicine, Karolinska University Hospital and Department of CLINTEC, Karolinska Institutet, Stockholm, Sweden 4 Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden 5 Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden 6 Unit of Biostatistics, Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden 7 Perioperative Medicine and Intensive Care, Karolinska University Hospital, Stockholm, Sweden 8 Coagulation Unit, Division of Hematology, Karolinska University Hospital, Stockholm, Sweden 9 Department of Clinical Sciences, Danderyd Hospital, Stockholm, Sweden NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. medRxiv preprint doi: https://doi.org/10.1101/2020.06.11.20128710; this version posted June 12, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. -

Serum Levels of True Insulin, C-Peptide and Proinsulin in Peripheral Blood of Patients with Cirrhosis
Diabetologia (1983) 25: 506-509 Diabetologia Springer-Verlag 1983 Serum Levels of True Insulin, C-Peptide and Proinsulin in Peripheral Blood of Patients with Cirrhosis T. Kasperska-Czy~ykowa 1, L. G. Heding 2 and A. Czy2yk 1 1Department of Gastroenterology and Metabolic Diseases, Medical Academy of Warsaw, Poland, and 2Novo Research Institute, Bagsvaerd, Denmark Summary. The levels of proinsulin, immunoreactive insulin, but the difference was less pronounced and only significant at true insulin (calculated from the difference, namely immuno- a few of the time points. The serum level of C-peptide was reactive insulin-proinsulin) and C-peptide were determined in very similar in both groups. These results emphasize that cir- the fasting state and during a 3-h oral glucose tolerance test af- rhosis is a condition in which the serum proinsulin level is ter administration of 100 g of glucose in 12 patients with cir- raised and that this hyperproinsulinaemia contributes greatly rhosis with normal oral glucose tolerance test (50 g) and in to the increased immunoreactive insulin levels observed in 12 healthy subjects serving as controls. In the patients with cir- patients with this disease. rhosis the serum levels of proinsulin and immunoreactive in- sulin were significantly higher in the fasting state and after Key words: Insulin, cirrhosis, C-peptide, proinsulin, oral glu- glucose loading than in the healthy subjects. The serum level cose tolerance test. of true insulin was also higher in the patients with cirrhosis, After the introduction of a radioimmunoassay for se- Patients and Methods rum (plasma) insulin determination (IRI) many authors reported raised levels of this hormone in the peripheral blood of patients with cirrhosis [1, 5-7, 9, 16-19]. -

Understanding Your Blood Test Lab Results
Understanding Your Blood Test Lab Results A comprehensive "Health Panel" has been designed specifically to screen for general abnormalities in the blood. This panel includes: General Chemistry Screen or (SMAC), Complete Blood Count or (CBC), and Lipid examination. A 12 hour fast from all food and drink (water is allowed) is required to facilitate accurate results for some of the tests in this panel. Below, is a breakdown of all the components and a brief explanation of each test. Abnormal results do not necessarily indicate the presence of disease. However, it is very important that these results are interpreted by your doctor so that he/she can accurately interpret the findings in conjunction with your medical history and order any follow-up testing if needed. The Bernards Township Health Department and the testing laboratory cannot interpret these results for you. You must speak to your doctor! 262 South Finley Avenue Basking Ridge, NJ 07920 www.bernardshealth.org Phone: 908-204-2520 Fax: 908-204-3075 1 Chemistry Screen Components Albumin: A major protein of the blood, albumin plays an important role in maintaining the osmotic pressure spleen or water in the blood vessels. It is made in the liver and is an indicator of liver disease and nutritional status. A/G Ratio: A calculated ratio of the levels of Albumin and Globulin, 2 serum proteins. Low A/G ratios can be associated with certain liver diseases, kidney disease, myeloma and other disorders. ALT: Also know as SGPT, ALT is an enzyme produced by the liver and is useful in detecting liver disorders. -

A Novel Perspective on the Biology of Bilirubin in Health and Disease
Opinion A Novel Perspective on the Biology of Bilirubin in Health and Disease 1,z 2,z, 3 Silvia Gazzin, Libor Vitek, * Jon Watchko, 4,5,6,7 1,8, Steven M. Shapiro, and Claudio Tiribelli * Unconjugated bilirubin (UCB) is known to be one of the most potent endogenous Trends antioxidant substances. While hyperbilirubinemia has long been recognized as Historically known for its toxicity but an ominous sign of liver dysfunction, recent data strongly indicate that mildly recently recognized as a powerful pro- tective molecule, BLB is gaining more elevated bilirubin (BLB) levels can be protective against an array of diseases attention due to its pleiotropic biomo- associated with increased oxidative stress. These clinical observations are lecular effects and those of the supported by new discoveries relating to the role of BLB in immunosuppression enzymes involved in BLB metabolism (the ‘Yellow Players’). and inhibition of protein phosphorylation, resulting in the modulation of intra- cellular signaling pathways in vascular biology and cancer, among others. Both heme oxygenase (HMOX) and bili- Collectively, the evidence suggests that targeting BLB metabolism could be verdin reductase (BLVR) (the main enzymes in BLB metabolism) act on considered a potential therapeutic approach to ameliorate a variety of numerous signaling pathways, with conditions. unsuspected biological consequences. The interconnections of such pathways highlight an incredibly complex biomo- From a Biological Waste Product to a Potent Biological Compound lecular network. Yellow player mole- UCB (see Glossary), the end product of the heme catabolic pathway, has long been recognized cules can have important physiological as a sign of liver dysfunction or a potential toxic factor causing severe brain damage in newborns. -

Community Lab Costs
Sickle Cell This tests for the genetic trait which may lead to sickle cell anemia. Osteoporosis Screening This uses ultrasound to screen people for low bone density or osteoporosis and is completed by painlessly scanning the heel. Because osteoporosis rarely causes signs or symptoms until it’s advanced, the National Osteoporosis Foundation recommends a bone density test if you are: • A woman older than age 65 or a man older than age 70 • A postmenopausal woman with at least one risk factor for osteoporosis Community Lab Costs • A man between age 50 and 70 who has at least one osteoporosis (greatly reduced rates) risk factor • Older than age 50 with a history of a broken bone • Taking medications, such as prednisone, aromatase inhibitors or Wellness Panel........................................................................ $20 Fasting anti-seizure drugs, that are associated with osteoporosis Includes screening for glucose, electrolytes, kidney, liver and • A postmenopausal woman who has recently stopped taking thyroid, plus complete blood count and lipid profile hormone therapy • A woman who experienced early menopause Diabetic Screening (A1c)...................................................... $5 The results of this test will indicate if you have or at risk for osteoporosis. Sickle Cell................................................................................ $6 If so, your doctor can offer treatment. PSA (Prostate Screening)...................................................... $8 Breathing Test This measures airflow and lung -
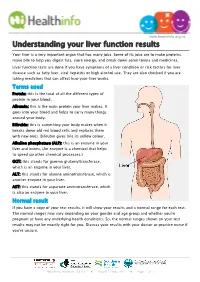
Understanding Your Liver Function Results
www.healthinfo.org.nz Understanding your liver function results Your liver is a very important organ that has many jobs. Some of its jobs are to make proteins, make bile to help you digest fats, store energy, and break down some toxins and medicines. Liver function tests are done if you have symptoms of a liver condition or risk factors for liver disease such as fatty liver, viral hepatitis or high alcohol use. They are also checked if you are taking medicines that can affect how your liver works. Terms used Protein: this is the total of all the different types of protein in your blood. Albumin: this is the main protein your liver makes. It goes into your blood and helps to carry many things around your body. Bilirubin: this is something your body makes when it breaks down old red blood cells and replaces them with new ones. Bilirubin gives bile its yellow colour. Alkaline phosphatase (ALP): this is an enzyme in your liver and bones. (An enzyme is a chemical that helps to speed up other chemical processes.) GGT: this stands for gamma glutamyltransferase, which is an enzyme in your liver. ALT: this stands for alanine aminotransferase, which is another enzyme in your liver. AST: this stands for aspartate aminotransferase, which is also an enzyme in your liver. Normal result If you have a copy of your test results, it will show your results and a normal range for each test. The normal ranges may vary depending on your gender and age group and whether you're pregnant or have any underlying health conditions. -

Jaundice and Your Baby
Jaundice and your baby What is jaundice? Jaundice is a common condition in newborn babies. It can give the skin or the white part of the eyes a yellow colour. The yellow comes from bilirubin in the blood. Before birth, the mother’s liver removes the bilirubin from the baby’s blood. After the baby is born, it takes a few days for the baby’s liver to get better at removing the bilirubin on its own. During this time, many babies develop jaundice. Jaundice can occur in a baby of any race or skin colour. Feeding your baby often (especially breastfeeding) in the first few hours and days after birth can help lower the risk of jaundice. This helps your baby pass more bowel movements (stools) and gives your baby’s liver the energy it needs to remove the bilirubin. Are some babies more likely to get jaundice? Yes, these circumstances can make jaundice more likely or make jaundice worse: Birth earlier than 2 weeks before the due date Bruising from a difficult delivery such as when forceps are used Baby’s blood type is different than the mother’s blood type Signs of jaundice within the first 24 hours after birth Baby has a sibling who was treated for jaundice Baby is not feeding well, especially if breastfeeding Baby has an infection Baby is of East Asian race (for example, Japanese, Chinese, Indonesian, Korean, Vietnamese or Cambodian) Baby has a family history of a genetic condition called G6PD deficiency Page - 1 Jaundice and your baby Is jaundice harmful? Most babies have mild jaundice, which is not harmful. -

TUBERCULOSIS Serodiagnostic Tests
TUBERCULOSIS Serodiagnostic Tests www.who.int/tb Policy Statement 2011 COMMERCIAL SERO DIAGNOSTIC TESTS FOR DIAGNOSIS OF ACTIVE TUBERCULOSIS CONCLUSION It is strongly recommended that these commercial tests not be used for the diagnosis of pulmonary and extra-pulmonary TB. Currently available commercial serodiagnostic tests (also referred to as serological tests) provide inconsistent and imprecise findings. There is no evidence that existing commercial serological assays improve patient outcomes, and high proportions of false-positive and false-negative results may have an adverse impact on the health of patients. EVI DENCE INTO POLICY AND PRACTICE RESULTS Sensitivity Pulmonary tuberculosis: Low sensitivity results in an 67 studies were reviewed, including 32 studies from low- and middle- unacceptably high number of income countries. The results demonstrated that sensitivity and patients being wrongly given the specificity from individual studies were highly variable. Pooled results 'all clear' (i.e. a false-negative). of the most widely used tests showed sensitivity at 76% and 59% and This can lead to them dying from specificity at 92% and 91% in smear-positive and smear-negative untreated tuberculosis, and the patients respectively. An evaluation by the TDR programme of 19 disease also being transmitted to rapid commercial tests, in comparison with culture plus clinical follow- others. up, showed similar variability with sensitivity values of 1% to 60% and Specificity specificity of 53% to 99%. Low specificity leads to an Extrapulmonary tuberculosis: unacceptably high number of 27 studies were reviewed, including 10 studies from low- and middle- patients being wrongly diagnosed income countries. The results demonstrated that sensitivity and with TB (i.e.