Metabolic Regulation of Heme Catabolism and Bilirubin Production
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Jaundice Protocol
fighting childhood liver disease Jaundice Protocol Early identification and referral of liver disease in infants fighting childhood liver disease 36 Great Charles Street Birmingham B3 3JY Telephone: 0121 212 3839 yellowalert.org childliverdisease.org [email protected] Registered charity number 1067331 (England & Wales); SC044387 (Scotland) The following organisations endorse the Yellow Alert Campaign and are listed in alphabetical order. 23957 CLDF Jaundice Protocol.indd 1 03/08/2015 18:25:24 23957 3 August 2015 6:25 PM Proof 1 1 INTRODUCTION This protocol forms part of Children’s Liver Disease Foundation’s (CLDF) Yellow Alert Campaign and is written to provide general guidelines on the early identification of liver disease in infants and their referral, where appropriate. Materials available in CLDF’s Yellow Alert Campaign CLDF provides the following materials as part of this campaign: • Yellow Alert Jaundice Protocol for community healthcare professionals • Yellow Alert stool colour book mark for quick and easy reference • Parents’ leaflet entitled “Jaundice in the new born baby”. CLDF can provide multiple copies to accompany an antenatal programme or for display in clinics • Yellow Alert poster highlighting the Yellow Alert message and also showing the stool chart 2 GENERAL AWARENESS AND TRAINING The National Institute of Health and Clinical Excellence (NICE) has published a clinical guideline on neonatal jaundice which provides guidance on the recognition, assessment and treatment of neonatal jaundice in babies from birth to 28 days. Neonatal Jaundice Clinical Guideline guidance.nice.org.uk cg98 For more information go to nice.org.uk/cg98 • Jaundice Community healthcare professionals should be aware that there are many causes for jaundice in infants and know how to tell them apart: • Physiological jaundice • Breast milk jaundice • Jaundice caused by liver disease • Jaundice from other causes, e.g. -

Serum Levels of True Insulin, C-Peptide and Proinsulin in Peripheral Blood of Patients with Cirrhosis
Diabetologia (1983) 25: 506-509 Diabetologia Springer-Verlag 1983 Serum Levels of True Insulin, C-Peptide and Proinsulin in Peripheral Blood of Patients with Cirrhosis T. Kasperska-Czy~ykowa 1, L. G. Heding 2 and A. Czy2yk 1 1Department of Gastroenterology and Metabolic Diseases, Medical Academy of Warsaw, Poland, and 2Novo Research Institute, Bagsvaerd, Denmark Summary. The levels of proinsulin, immunoreactive insulin, but the difference was less pronounced and only significant at true insulin (calculated from the difference, namely immuno- a few of the time points. The serum level of C-peptide was reactive insulin-proinsulin) and C-peptide were determined in very similar in both groups. These results emphasize that cir- the fasting state and during a 3-h oral glucose tolerance test af- rhosis is a condition in which the serum proinsulin level is ter administration of 100 g of glucose in 12 patients with cir- raised and that this hyperproinsulinaemia contributes greatly rhosis with normal oral glucose tolerance test (50 g) and in to the increased immunoreactive insulin levels observed in 12 healthy subjects serving as controls. In the patients with cir- patients with this disease. rhosis the serum levels of proinsulin and immunoreactive in- sulin were significantly higher in the fasting state and after Key words: Insulin, cirrhosis, C-peptide, proinsulin, oral glu- glucose loading than in the healthy subjects. The serum level cose tolerance test. of true insulin was also higher in the patients with cirrhosis, After the introduction of a radioimmunoassay for se- Patients and Methods rum (plasma) insulin determination (IRI) many authors reported raised levels of this hormone in the peripheral blood of patients with cirrhosis [1, 5-7, 9, 16-19]. -

A Novel Perspective on the Biology of Bilirubin in Health and Disease
Opinion A Novel Perspective on the Biology of Bilirubin in Health and Disease 1,z 2,z, 3 Silvia Gazzin, Libor Vitek, * Jon Watchko, 4,5,6,7 1,8, Steven M. Shapiro, and Claudio Tiribelli * Unconjugated bilirubin (UCB) is known to be one of the most potent endogenous Trends antioxidant substances. While hyperbilirubinemia has long been recognized as Historically known for its toxicity but an ominous sign of liver dysfunction, recent data strongly indicate that mildly recently recognized as a powerful pro- tective molecule, BLB is gaining more elevated bilirubin (BLB) levels can be protective against an array of diseases attention due to its pleiotropic biomo- associated with increased oxidative stress. These clinical observations are lecular effects and those of the supported by new discoveries relating to the role of BLB in immunosuppression enzymes involved in BLB metabolism (the ‘Yellow Players’). and inhibition of protein phosphorylation, resulting in the modulation of intra- cellular signaling pathways in vascular biology and cancer, among others. Both heme oxygenase (HMOX) and bili- Collectively, the evidence suggests that targeting BLB metabolism could be verdin reductase (BLVR) (the main enzymes in BLB metabolism) act on considered a potential therapeutic approach to ameliorate a variety of numerous signaling pathways, with conditions. unsuspected biological consequences. The interconnections of such pathways highlight an incredibly complex biomo- From a Biological Waste Product to a Potent Biological Compound lecular network. Yellow player mole- UCB (see Glossary), the end product of the heme catabolic pathway, has long been recognized cules can have important physiological as a sign of liver dysfunction or a potential toxic factor causing severe brain damage in newborns. -
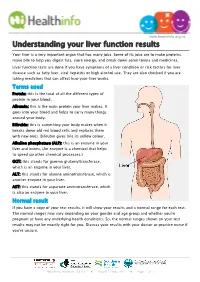
Understanding Your Liver Function Results
www.healthinfo.org.nz Understanding your liver function results Your liver is a very important organ that has many jobs. Some of its jobs are to make proteins, make bile to help you digest fats, store energy, and break down some toxins and medicines. Liver function tests are done if you have symptoms of a liver condition or risk factors for liver disease such as fatty liver, viral hepatitis or high alcohol use. They are also checked if you are taking medicines that can affect how your liver works. Terms used Protein: this is the total of all the different types of protein in your blood. Albumin: this is the main protein your liver makes. It goes into your blood and helps to carry many things around your body. Bilirubin: this is something your body makes when it breaks down old red blood cells and replaces them with new ones. Bilirubin gives bile its yellow colour. Alkaline phosphatase (ALP): this is an enzyme in your liver and bones. (An enzyme is a chemical that helps to speed up other chemical processes.) GGT: this stands for gamma glutamyltransferase, which is an enzyme in your liver. ALT: this stands for alanine aminotransferase, which is another enzyme in your liver. AST: this stands for aspartate aminotransferase, which is also an enzyme in your liver. Normal result If you have a copy of your test results, it will show your results and a normal range for each test. The normal ranges may vary depending on your gender and age group and whether you're pregnant or have any underlying health conditions. -

Association Between Serum Bilirubin and Albuminuria in Type 2 Diabetes Mellitus and Diabetic Nephropathy
Original Research Article DOI: 10.18231/2394-6377.2018.0048 Association between serum bilirubin and albuminuria in type 2 diabetes mellitus and diabetic nephropathy Suryapriya Rajendran1, Manju M.2,*, Sasmita Mishra3, Rakesh Kumar4 1,4Assistant Professor, 2Associate Professor, 3Professor and HOD, Dept. of Biochemistry, 4Dept. of General Medicine, Aarupadai Veedu Medical College and Hospital, Puducherry, India. *Corresponding Author: Email: [email protected] Received: 16th February, 2018 Accepted: 20th March, 2018 Abstract Introduction: Diabetic nephropathy develops due to oxidative stress and inflammation resulting from chronic hyperglycemia. Bilirubin, a product of heme catabolism is found to have antioxidant and anti-inflammatory properties. Though previous studies have examined the relationship between total bilirubin and diabetic nephropathy, very few studies have focused on indirect and direct bilirubin levels. Hence, the present study aimed to compare serum bilirubin (total, indirect and direct) levels between non- diabetics, type 2 diabetics and diabetic nephropathy subjects and also to correlate albuminuria with serum bilirubin in type 2 diabetics and diabetic nephropathy subjects. Materials and Methods: 50 non-diabetics, 50 type 2 diabetics and 50 diabetic nephropathy subjects were included in the study. Fasting blood glucose, HbA1C, serum bilirubin (total, indirect and direct), serum creatinine, urine microalbumin and urine creatinine were measured. Estimated glomerular filtration rate (eGFR) and urine albumin creatinine ratio (ACR) was calculated. Results: Total bilirubin, direct and indirect bilirubin were significantly decreased in type 2 diabetics and diabetic nephropathy subjects compared to non-diabetics. Total bilirubin and indirect bilirubin were also significantly decreased in diabetic nephropathy subjects compared to type 2 diabetics. Total bilirubin, direct and indirect bilirubin showed significant negative correlation with albuminuria (Urine ACR) in type 2 diabetics and diabetic nephropathy subjects. -

Cholestasis and Neonatal Hypoglycaemia
MOJ Clinical & Medical Case Reports Case Report Open Access Cholestasis and neonatal hypoglycaemia Abstract Volume 10 Issue 4 - 2020 Glucose is essential as the main brain energy source, hypoglycemia is dangerous and may Nahla Kamel Gaballa,1 Hossam Bassiouny,2 cause neuronal damage as well as siezers and acidosis. The undetectable hypoglycemia has Tahany Abdel hameed,2 Mahmoud EL serious effects if passed unnoticed specially in infants who cannot explain their sensation 3 4 1 and complain , any abnormal clinical or laboratory response should be addressed as a Moniar, Hazem Zakaria, Eman Awad, Heba 5 special entity and dealing with as if a complain that must be investigated. Abd Alla 1Lecturer of Anesthesia and intensive care department, national liver institute, Menoufia University, Egypt 2Lecturer of pediatric hepatology department, Menoufia University, Egypt 3Assistant Lecturer of Anesthesia and intensive care department, Menoufia University, Egypt 4Associate professor of hepatopancreatobiliary, surgical department national liver institute, Menoufia University, Egypt 5Lecturer of clinical pathology and hematology, clinical pathology department, Menoufia University, Egypt Correspondence: Nahla Kamel Gaballa, Lecturer of Anesthesia and intensive care department, national liver institute, Menoufia University, Egypt, Email Received: June 23, 2020 | Published: July 14, 2020 Case report -2.5ml/kg was given(short term hypoglycemia treatment) ,starting the operation and he was diagnosed as type 2 obstruction, the next hourly An infant 83 days old for Kassi operation, 4.5kg, normal growing reading of RBS was 77mg/dl and again second bolus of Glucose parameters and reactive, without mother complain regarding feeding 10% was given, then starting Glucose 10% infusion 5mg/kg/min, or suckling, normal birth history, progressive jaundice after birth and the operation lasted for 4hours including hernial repair and caudal history of blood transfusion after Hb drop in rural pediatric department. -

Guideline for the Evaluation of Cholestatic Jaundice
CLINICAL GUIDELINES Guideline for the Evaluation of Cholestatic Jaundice in Infants: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition ÃRima Fawaz, yUlrich Baumann, zUdeme Ekong, §Bjo¨rn Fischler, jjNedim Hadzic, ôCara L. Mack, #Vale´rie A. McLin, ÃÃJean P. Molleston, yyEzequiel Neimark, zzVicky L. Ng, and §§Saul J. Karpen ABSTRACT Cholestatic jaundice in infancy affects approximately 1 in every 2500 term PREAMBLE infants and is infrequently recognized by primary providers in the setting of holestatic jaundice in infancy is an uncommon but poten- physiologic jaundice. Cholestatic jaundice is always pathologic and indicates tially serious problem that indicates hepatobiliary dysfunc- hepatobiliary dysfunction. Early detection by the primary care physician and tion.C Early detection of cholestatic jaundice by the primary care timely referrals to the pediatric gastroenterologist/hepatologist are important physician and timely, accurate diagnosis by the pediatric gastro- contributors to optimal treatment and prognosis. The most common causes of enterologist are important for successful treatment and an optimal cholestatic jaundice in the first months of life are biliary atresia (25%–40%) prognosis. The Cholestasis Guideline Committee consisted of 11 followed by an expanding list of monogenic disorders (25%), along with many members of 2 professional societies: the North American Society unknown or multifactorial (eg, parenteral nutrition-related) causes, each of for Gastroenterology, Hepatology and Nutrition, and the European which may have time-sensitive and distinct treatment plans. Thus, these Society for Gastroenterology, Hepatology and Nutrition. This guidelines can have an essential role for the evaluation of neonatal cholestasis committee has responded to a need in pediatrics and developed to optimize care. -
Interpreting Liver Function Tests CONTENTS
A CASE-BASED SERIES ON PRACTICAL PATHOLOGY FOR GPs FEBRUARY 2020 Interpreting liver function tests CONTENTS: • Dealing with abnormal LFTs in the asymptomatic patient • Diagnosing Gilbert’s syndrome © The Royal College of • Establishing the source of raised ALP Pathologists of Australasia 2 Authors: Dr Melissa Gillett Dr Rebecca Brereton MBBS, FRACP, FRCPA, MAACB Specialist Chemical Pathologist, Chemical Pathologist, Fiona Stanley Hospital Network Fiona Stanley Hospital Network Laboratory, PathWest Laboratory Laboratory, PathWest Laboratory Medicine, Murdoch, WA Medicine, Murdoch, WA Common Sense Pathology is developed by the Royal College of Pathologists of Australasia and supported by Australian Doctor Group. © 2020 Royal College of Pathologists of Australasia www.rcpa.edu.au CEO: Dr Debra Graves Email: [email protected] While the views expressed are those of the authors, modified by expert reviewers, they are not necessarily held by the College. Published by Australian Doctor Group Level 2, 26-32 Pyrmont Bridge Road, Pyrmont NSW 2009 Ph: 1300 360 126 Email: [email protected] Website: www.australiandoctorgroup.com.au ACN: 615 959 914 ABN: 94 615 959 914 ISSN: 1039-7116 The views expressed in this publication are not necessarily those of Australian Doctor Group. This issue is produced and owned by the Royal College of Pathologists of Australasia and distributed by Australian Doctor Group. Common Sense Pathology Editor: Dr Steve Flecknoe-Brown Email: [email protected] Editor: Dr Karley Heyworth Email: [email protected] Sub-editor: Lesley Hoye Email: [email protected] Graphic Designer: Kate O’Dea Email: [email protected] For an electronic version of this issue, please visit www.howtotreat.com.au You can also visit the Royal College of Pathologists of Australasia’s website at www.rcpa.edu.au Click on Library and Publications, then Common Sense Pathology. -

Low Glycated Hemoglobin and Liver Disease in the U.S. Population
Epidemiology/Health Services Research BRIEF REPORT Low Glycated Hemoglobin and Liver Disease in the U.S. Population 1,2 1,2,3 ANDREA L. CHRISTMAN, BA JEANNE M. CLARK, MD, MPH (n = 3,771). To assess the continuous asso- 1,2 1,2 MARIANA LAZO, MD, PHD ELIZABETH SELVIN, PHD, MPH ciations, we fit restricted cubic splines (9). We conducted a sensitivity analysis exclud- ing participants with anemia (hemoglobin d , OBJECTIVE To characterize the association of low HbA1c values ( 4.0%) with liver ,13.5g/dLinmenand,12.0 g/dL in enzymes and steatosis. women), presumed iron overload (serum d transferrin saturation .44% and serum RESEARCH DESIGN AND METHODS Cross-sectional study of 12,533 participants , without diabetes aged ,20 years in the Third National Health and Nutrition Examination Survey ferritin 10 ng/mL), or hepatitis B or C (1988–1994). Logistic regression models were adjusted for demographic, lifestyle, and health (n = 11,593, after exclusions). To compare status variables. low HbA1c to low fasting glucose, we mod- eled the 1st percentile versus the rest of the RESULTSdHbA values ranged from 3.2 to 15.7%, and 84 participants had HbA ,4.0% in 1c 1c values for HbA1c (cutpoint, 4.0%) and fast- the population (mean age 44, 52% female, 15% black or Hispanic). We observed J-shaped ing blood glucose (cutpoint, 73.4 mg/dL) in associations between HbA1c and liver enzymes and hepatic steatosis. In adjusted models, HbA1c the morning fasting subsample ( = 8,747). ,4.0% was strongly associated with elevated alanine aminotransferase (OR 3.62 [95% CI 1.09– n 12.02]) and aspartate aminotransferase (6.80 [2.99–15.43]). -

Bilirubin Test? This Is a Blood Test That Measures the Total Amount of Bilirubin in the Blood
Midlands Family Medicine 611 West Francis St. Suite 100 North Platte, NE 69101 Phone: (308) 534-2532 Fax: (308) 534-6615 Education Bilirubin (Total) Test What is the total bilirubin test? This is a blood test that measures the total amount of bilirubin in the blood. Bilirubin is released into the blood when red blood cells break down. The liver uses bilirubin to make bile. Normally there is only a small amount of bilirubin in the blood. High levels may be caused by liver or blood problems. When the bilirubin level rises, it causes the skin and whites of the eyes to become yellow. This change to yellow is called jaundice. Why is this test done? This test may be done to: See how well your liver is working. Check for blockage of bile in the bile duct, gallbladder, or liver. Bile, which contains bilirubin, is normally released into the intestines through the bile duct. If the bile duct becomes blocked, or if there is blockage of bile in the gallbladder or liver, the bilirubin level rises in the blood since it is no longer released into the intestines. Check for certain blood problems, such as some types of anemia. Blood problems may cause a high level of bilirubin because more red blood cells are breaking down than normal. How do I prepare for this test? Do not eat or drink anything for several hours before the test. Other liver tests that are usually measured at the same time as bilirubin are not as accurate if you have eaten. Ask your health care provider how long you should fast. -
Understanding Your Lab Results: Comprehensive Metabolic Screening Panel
Understanding Your Lab Results: Comprehensive Metabolic Screening Panel CHOLESTEROL and TRIGLYCERIDES are fats necessary for normal cell function. However, elevated levels of these fats have been associated with an increased risk of developing coronary disease, arteriosclerosis, and heart attack. A patient’s dietary status, medications, presence of illness, lifestyle, and family history may represent factors influencing cholesterol levels. The signifi- cance of cholesterol levels should be determined within the context of each individual patient. If your cholesterol level is 200 mg/dl or greater, please consult your physician. Triglyceride levels greater than 150 mg/dl, in a true fasting specimen, are considered elevated. In this case, please consult your physician. HDL (High-Density Lipoprotein) CHOLESTEROL, commonly known as the “GOOD” cholesterol, picks up cholesterol and transports it for removal from the body. The higher the HDL value, the lower the risk of developing coronary disease, arterioscle- rosis, and heart attack. LDL (Low-Density Lipoprotein) CHOLESTEROL, commonly known as the “BAD” cholesterol, picks up cholesterol and transports it to the cells of the body for storage. Desirable LDL levels are less than 130 mg/dl. The higher the LDL value, the greater the risk of developing coronary disease, arteriosclerosis, and heart attack. CHOLESTEROL/HDL RATIO is a calculation used to predict an increased or decreased risk of cardiovascular disease relative to a normal. The higher the ratio, the greater the risk of developing coronary disease, arteriosclerosis, and heart attack. GLUCOSE, commonly called a blood sugar, is the transport form of carbohydrates in the body as they move to storage or to utiliza- tion. -
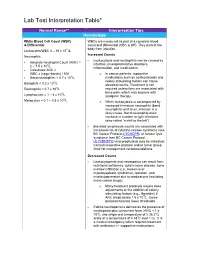
Lab Test Interpretation Table*
Lab Test Interpretation Table* Normal Range** Interpretation Tips Hematology White Blood Cell Count (WBC) WBCs are measured as part of a complete blood & Differential count and differential (CBC & diff). They protect the body from infection. Leukocytes/WBC 4 – 10 x 109 /L Increased Counts Neutrophils - Leukocytosis and neutrophilia can be caused by Absolute Neutrophil Count (ANC) = - infection, myeloproliferative disorders, 2 – 7.5 x 109/L inflammation, and medications. - Calculated ANC = WBC x (segs+bands) / 100 o In cancer patients, supportive - Band neutrophils: < 0.7 x 109/L medications such as corticosteroids and 9 colony stimulating factors can cause Basophils < 0.2 x 10 /L elevated counts. Treatment is not Eosinophils < 0.7 x 109/L required unless they are associated with 9 bone pain, which may improve with Lymphocytes = 1 – 4 x 10 /L analgesic therapy. 9 Monocytes = 0.1 – 0.8 x 10 /L o When leukocytosis is accompanied by increased immature neutrophils (band neutrophils) and fever, infection is a likely cause. Band neutrophils often increase in number to fight infections (also called “a shift to the left”). - Elevated lymphocyte counts are associated with increased risk of cytokine-release syndrome (see BC Cancer Protocol LYCHOPR) or tumour lysis syndrome (see BC Cancer Protocol ULYVENETO) and prophylaxis may be indicated. Consult respective protocol and/or tumor group chair for management recommendations. Decreased Counts - Leukocytopenia and neutropenia can result from nutritional deficiency, autoimmune disease, bone marrow infiltration (i.e., leukemia or myelodysplastic syndrome), radiation, and myelosuppression due to medications (including many cancer drugs). o Many treatment protocols require dose adjustments or the addition of colony stimulating factors (e.g., filgrastim) if ANC drops below 1.5 x 109/L.