Fructosamine Is a Valuable Marker for Glycemic Control And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
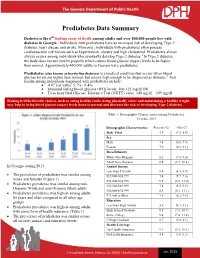
2015 Prediabetes Data Summary
The Georgia Department of Public Health Prediabetes Data Summary Diabetes is the 6th leading cause of death among adults and over 800,000 people live with diabetes in Georgia.1 Individuals with prediabetes have an increased risk of developing Type 2 diabetes, heart disease and stroke. Moreover, individuals with prediabetes often possess cardiovascular risk factors such as hypertension, obesity and high cholesterol. Prediabetes almost always occurs among individuals who eventually develop Type 2 diabetes.2 In Type 2 diabetes, the body does not use insulin properly which causes blood glucose (sugar) levels to be higher than normal. Approximately 450,000 adults in Georgia have prediabetes. Prediabetes (also known as borderline diabetes) is a medical condition that occurs when blood glucose levels are higher than normal, but are not high enough to be diagnosed as diabetes.3 Test results among individuals diagnosed with prediabetes include: A1C test value: 5.7% - 6.4% Impaired fasting blood glucose (IFG) levels: 100-125 mg/dl OR Two- hour Oral Glucose Tolerance Test (OGTT) value: 140 mg/dl – 199 mg/dl Making healt hy lifestyle choices, such as eating healthy foods, being physically active and maintaining a healthy weight, may help to bring blood glucose (sugar) levels down to normal and decrease the risk of developing Type 2 diabetes Table 1. Demographic Characteristics among Prediabetics, Georgia, 2013 Demographic Characteristics Percent (%) 95% CI State Total 7.9 (7.2, 8.9) Sex Male 7.8 (6.8, 9.5) Female 7.9 (6.9, 9.1) Race/Ethnicity -

Genetically Determined Hypoalbuminemia As a Risk Factor for Hypertension: Instrumental Variable Analysis Jong Wook Choi1, Joon‑Sung Park2* & Chang Hwa Lee2*
www.nature.com/scientificreports OPEN Genetically determined hypoalbuminemia as a risk factor for hypertension: instrumental variable analysis Jong Wook Choi1, Joon‑Sung Park2* & Chang Hwa Lee2* Hypoalbuminemia is associated with vascular endothelial dysfunction and the development of chronic cardiovascular diseases. However, the relationship between serum albumin concentration and blood pressure changes remains controversial. Community‑based longitudinal cohort data collected from Korean Genome and Epidemiology Study were used in this study. Hypoalbuminemia was defned as a serum albumin concentration of ≤ 4.0 g/dL. A total of 4325 participants were categorized into control (n = 3157) and hypoalbuminemia (n = 1168) groups. Serum albumin had a non‑linear relationship with the risk of hypertension development. A genome‑wide association study revealed 71 susceptibility loci associated with hypoalbuminemia. Among susceptibility loci, genetic variations at rs2894536 in LOC107986598 and rs10972486 in ATP8B5P were related to elevated blood pressure. Serum albumin (HR = 0.654, 95% CI 0.521–0.820) and polymorphisms of rs2894536 (HR = 1.176, 95% CI 1.015–1.361) and rs10972486 (HR = 1.152, 95% CI 1.009–1.316) were signifcant predictors of hypertension development. Increased albumin concentration instrumented by 2 hypoalbuminemia‑associated SNPs (rs2894536 and rs10972486) was associated with decreased HRs for hypertension development (HR = 0.762, 95% CI 0.659–0.882 and HR = 0.759, 95% CI 0.656–0.878). Our study demonstrated that genetically determined hypoalbuminemia is a signifcant predictor of incipient hypertension. Albumin, one of the major serum proteins, has multiple important physiological functions involving stabilization of plasma colloid osmotic pressure, transportation of diverse substances, and signifcant antioxidant activity, and its concentration is fnely regulated by various systems in the physiologic state 1. -

Diabetes Control in Thyroid Disease
In Brief Thyroid disease is commonly found in most types of diabetes. This article defines the prevalence of thyroid disease in diabetes and elucidates through case studies the assessment, diagnosis, and clinical management of thyroid disease in diabetes. Diabetes Control in Thyroid Disease Thyroid disease is a pathological state abnormality. Several studies, including that can adversely affect diabetes con- the Colorado study, have documented trol and has the potential to negative- a higher prevalence of thyroid disease Jennal L. Johnson, MS, RNC, FNP, ly affect patient outcomes. Thyroid in women, with prevalence rates rang- BC-ADM, CDE disease is found commonly in most ing from 4 to 21%, whereas the rate in forms of diabetes and is associated men ranges from 2.8 to 16%.1 with advanced age, particularly in Thyroid disease increases with age. In type 2 diabetes and underlying the Colorado study, the 18-year-olds autoimmune disease in type 1 dia- had a prevalence rate of 3.5% com- betes. This article defines the preva- pared with a rate of 18.5% for those lence of thyroid disease in diabetes, ≥ 65 years of age. discusses normal physiology and The prevalence of thyroid disease in screening recommendations for thy- diabetes has been estimated at 10.8%,2 roid disease, and elucidates through with the majority of cases occurring as case studies the assessment, diagnosis, hypothyroidism (~ 30%) and subclini- and clinical management of thyroid cal hypothyroidism (~ 50%).2 Hyper- disease and its impact on diabetes. thyroidism accounts for 12%, and postpartum thyroiditis accounts for Thyroid Disease Prevalence 11%.2 Of the female patients with The prevalence of thyroid disease in type 1 diabetes, 30% have thyroid dis- the general population is estimated to ease, with a rate of postpartum thy- be 6.6%, with hypothyroidism the roid disease three times that of the most common malady.1 Participants normal population. -

Glycated Hemoglobin and Glycated Albumin in Patients with Diabetes
Kitajima et al. Renal Replacement Therapy (2020) 6:10 https://doi.org/10.1186/s41100-020-0260-5 RESEARCH Open Access Glycated hemoglobin and glycated albumin in patients with diabetes undergoing hemodiafiltration Yukie Kitajima1*, Shunichiro Urabe2, Takashi Hosono2, Satoshi Yoshikawa3, Yuzuru Sato3 and Toru Hyodo2 Abstract Background: Online hemodiafiltration (OHDF), which results in high albumin leakage, is now widely used in Japan for dialysis, since the national insurance system began reimbursing its costs in 2012. Glycated albumin (GA) levels are affected by albumin leakage into effluent dialysate fluid. Therefore, GA levels in patients requiring diabetes- related dialysis undergoing OHDF require monitoring. However, there have been no previous reports on glycemic control indicators of patients with diabetes undergoing OHDF. We aimed to develop a glycemic control index for patients requiring diabetes-related dialysis undergoing OHDF. Methods: This study comprised 133 diabetic patients undergoing OHDF. We examined the correlation between GA and glycated hemoglobin (HbA1c) levels. We analyzed effluent dialysate fluid samples from 41 patients classified into 3 groups, namely, group A, non-protein-leaking OHDF (n = 20); group B, protein-leaking OHDF (n = 14); and group C, highly efficient protein-leaking OHDF (n = 7). We examined the association between GA and HbA1c levels in each group and among patients. Results: A significant positive correlation was observed between GA and HbA1c levels (r = 0.562, p < 0.0001). There was no significant correlation between pre-dialysis blood glucose levels and HbA1c or GA levels as observed on regular blood tests performed under non-fasting conditions. Patients were classified into 2 groups based on their mean albumin levels (3.4 g/dL cutoff). -

Glossary of Common Diabetes Terms
Glossary of Common Diabetes Terms A1C: a test that reveals exactly how well your blood sugar (glucose) has been controlled over the previous three months Beta cells: cells found in the pancreas that make insulin Blood glucose: also known as blood sugar, glucose comes from food and is then carried through the blood to deliver energy to cells Blood glucose meter: a small medical device used to check blood glucose levels Blood glucose monitoring: the simple blood test used to check the amount of glucose in the blood; a tiny drop of blood, taken by pricking a finger, is placed on a test strip and inserted in the meter for reading Diabetes: the shortened name for diabetes mellitus, the condition in which the pancreas doesn’t produce enough insulin or your body is unable to use insulin to move glucose into cells of the body Diabetic retinopathy: the eye disease that occurs in someone with diabetes when the small blood vessels of the retina become swollen and leak liquid into the retina, blurring vision; it can sometimes lead to blindness Gestational diabetes: the diabetes some women develop during pregnancy; it typically subsides after the baby is delivered, but many women who have had gestational diabetes may develop type 2 diabetes later in life Glucagon: the hormone that is injected into a person with diabetes to raise their blood glucose level when it’s very low (hypoglycemia) Glucose: blood sugar that gives energy to cells Hyperglycemia: also known as high blood glucose, this condition occurs when your blood glucose level is too high; -

CANINE INSULINOMA: DIAGNOSIS, TREATMENT, & STAGING Eliza Reiss Grant, DVM, and Kristine E
Peer Reviewed PRACTICAL ONCOLOGY CANINE INSULINOMA: DIAGNOSIS, TREATMENT, & STAGING Eliza Reiss Grant, DVM, and Kristine E. Burgess, DVM, Diplomate ACVIM (Oncology) Tufts University An insulinoma is a malignant pancreatic tumor that DIAGNOSIS inappropriately secretes excessive insulin, resulting in Aside from a histologic confirmation of insulinoma, profound hypoglycemia.1 no currently available diagnostic test provides a de- Pancreatic tumors are classified as: finitive diagnosis of insulinoma. Existing techniques • Exocrine, which includes adenocarcinomas of may help increase suspicion for an insulin-secreting ductular or acinar origin tumor but, with most diagnostic testing, it is im- • Endocrine, which arise from the islets of perative to interpret all results in the context of the Langerhans. coexisting clinical signs. Insulinomas are functional neuroendocrine tumors that originate in the beta cells of the islets Differential Diagnosis of Langerhans.1 A complete work-up, including careful patient history, physical examination, bloodwork, and PRESENTATION diagnostic imaging tests, should be performed to Signalment rule out other causes of hypoglycemia, such as Any breed of dog can be affected, but large sepsis, hepatic failure, adrenal cortical insufficiency, breeds tend to be overrepresented.1 While, in toxin ingestion, and other forms of neoplasia. humans, insulinomas affect females far more frequently than males, there is no apparent sex Laboratory Tests predilection in dogs.1-3 Dogs also commonly Blood Glucose present with a malignant variant, while humans A simple fasting blood glucose level of less than often have a benign adenoma (80%).1 Insulino- 40 mg/dL can suggest hyperinsulinemia, although ma is rare in cats.4 careful monitoring of a fasted dog with suspected insulinoma is strongly recommended due to high Clinical Signs risk for seizure activity. -

Bilirubin As a New Biomarker of Diabetes and Its Microvascular
Analytica & l B y i tr o s c i h m e m e h i Biochemistry & s c t Nishimura, et al., Biochem Anal Biochem 2016, 5:1 o r i y B DOI: 10.4172/2161-1009.1000245 ISSN: 2161-1009 Analytical Biochemistry Commentary Open Access Bilirubin as a New Biomarker of Diabetes and its Microvascular Complications Takeshi Nishimura*, Masami Tanaka, Risa Sekioka and Hiroshi Itoh Department of Internal Medicine, School of Medicine, Keio University, Tokyo, Japan *Corresponding author: Takeshi Nishimura, Department of Internal Medicine, School of Medicine, Keio University 35 Shinanomachi, Shinjuku-ku, Tokyo 160-8582, Japan, Tel: 81-3-5363-3797; Fax: 81-3-3359-2745; E-mail: [email protected] Rec date: Feb 06, 2016; Acc date: Feb 12, 2016; Pub date: Feb 15, 2016 Copyright: © 2016 Nishimura T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Commentary elucidate the pathophysiological role of bilirubin in type 1 DM, our results should be verified in large-scale, prospective studies. Hyperglycemia is the hallmark of diabetes mellitus [DM]; it activates certain biochemical pathways leading to micro- and macrovascular complications in diabetic patients [1]. Moreover, References hyperglycemia generates oxidative stress and causes free radical- 1. Takayanagi R, Inoguchi T, Ohnaka K (2011) Clinical and experimental mediated lipid peroxidation [2,3]. In turn, the oxidative stress causes evidence for oxidative stress as an exacerbating factor of diabetes mellitus. -

A History of Diabetes NYSNA Continuing Education the New
A History of Diabetes NYSNA Continuing Education The New York State Nurses Association is accredited as a provider of continuing nursing education by the American Nurses Credentialing Center’s Commission on Accreditation. This course has been awarded 1.2 contact hours. All American Nurses Credentialing Center (ANCC) accredited organizations' contact hours are recognized by all other ANCC accredited organizations. Most states with mandatory continuing education requirements recognize the ANCC accreditation/approval system. Questions about the acceptance of ANCC contact hours to meet mandatory regulations should be directed to the Professional licensing board within that state. NYSNA has been granted provider status by the Florida State Board of Nursing as a provider of continuing education in nursing (Provider number 50-1437). A History of Diabetes 1 © 2004 NYSNA, all rights reserved. Material may not be reproduced without written permission. How to Take This Course Please take a look at the steps below; these will help you to progress through the course material, complete the course examination and receive your certificate of completion. 1. REVIEW THE OBJECTIVES The objectives provide an overview of the entire course and identify what information will be focused on. Objectives are stated in terms of what you, the learner, will know or be able to do upon successful completion of the course. They let you know what you should expect to learn by taking a particular course and can help focus your study. 2. STUDY EACH SECTION IN ORDER Keep your learning "programmed" by reviewing the materials in order. This will help you understand the sections that follow. -

Food Fact Sheet
Food Fact Sheet Diabetes - Type 2 What is Type 2 diabetes? Diabetes is a condition where the amount of glucose (sugar) in your blood is too high because your body cannot use it properly. In Type 2 diabetes this happens because your pancreas doesn’t produce enough of the hormome insulin (that helps glucose enter body cells) and/or the insulin that is produced does not work correctly (insulin resistance). The importance of good blood glucose control 2 Reduce your portion sizes to help you reduce and People with Type 2 diabetes need to control their maintain a healthy weight. blood glucose. It is also important to look after your A portion is: heart health. Making changes to your lifestyle, diet and • a fist size of potatoes, bread, pasta or other activity level can be key to reducing the risk of diabetes starchy carbohydrates causing you problems now and in the future. • a palm size of meat/fish or poultry What can you eat? • two handfuls of vegetables or salad • a cupped-handful of fruit People with diabetes should eat a healthy diet, the • top of your thumb size of oil or fat spread. same as somebody without diabetes. It should be low Try using a smaller plate, filling half of your plate with in saturated fat, high in fibre and include a variety of vegetables and avoiding second helpings. fruit and vegetables. 3 Carbohydrates are used for energy so include some The eatwell guide in your diet each day. Opt for wholegrain options, fruits and vegetables, beans, pulses, low fat milk and The eatwell guide represents the main food groups and yoghurt. -

Comparative Evaluation of Fructosamine and Hba1c As a Marker of Glycemic Control in Type 2 Diabetes: a Hospital Based Study
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Original Research Article Comparative Evaluation of Fructosamine and HbA1c as a Marker of Glycemic Control in Type 2 Diabetes: A Hospital Based Study Dr. Jyoti Goyal1, Dr. Nibhriti Das2, Dr. Navin Kumar3, Ms. Seema Raghav4, Dr. Paramjeet Singh Bhatia5, Dr. Karunesh Prasad Singh6, Dr. Sabari Das7 1DNB, Department of Internal Medicine, Nayati Healthcare and Research Centre, Mathura, India- 281003, 2Ex-Director of Laboratory services and Additional Dean Research and Academics, Nayati Healthcare and Research Centre, Mathura, India- 281003, 3Ph.D, Biostatistitian, Department of Biostatistics, Nayati Healthcare and Research Centre, Mathura, India-281003. 4M.Sc., Certified Diabetes Educator, Department of Internal Medicine, Nayati Healthcare and Research Centre, Mathura, India-281003. 5MD, Department of Internal Medicine, Nayati Healthcare and Research Centre, Mathura, India-281003. 6MD Physician, Department of Internal Medicine, Nayati Healthcare and Research Centre, Mathura, India-281003. 7Department of Laboratory Medicine, Nayati Healthcare and Research Centre, Mathura, India- 281003, Corresponding Author: Dr. Jyoti Goyal ABSTRACT Introduction: Management of type 2 diabetes revolves around achievement of target glycemic control with the help of antidiabetic drugs or insulin. There are various markers for measurement of glyceamic control like HbA1c, Mean Blood Glucose and fructosamine levels. Though HbA1c is a well validated standard method for assessment of glycemic control but it has also got certain limitations. Fructosamine, a less explored method may be used as an alternative marker for an assessment of glycemic control in cases where HbA1c is unreliable or unavailable. The objective of this study is to compare the fructosamine levels with HbA1c in assessment of glycemic control in type 2 diabetics so as to assess the utility of fructosamine as an alternative marker for evaluation of glucose control. -

Diabetes Is a Disease in Which the Body's Ability to Produce Or Respond
Early Signs and Symptoms of diabetes: Early symptoms of diabetes, especially type 2 diabetes, can be subtle or seemingly harmless. Over time, however, you may Diabetes is a disease in which the develop diabetes complications, even if you body’s ability to produce or respond to haven't had diabetes symptoms. In the the hormone insulin is impaired, United States alone, more than 8 million resulting in abnormal metabolism of people have undiagnosed diabetes, Treatments: carbohydrates and elevated levels of according to the American Diabetes Association. Understanding possible glucose (sugar) in the blood. • Insulin therapy diabetes symptoms can lead to early • Oral medications diagnosis and treatment and a lifetime of Diabetes can be broken down into • better health. If you're experiencing any of Diet changes two types, Type 1 and Type 2. Type 1 • Exercise diabetes involves the the following diabetes signs and symptoms, see your doctor. body attacking itself by The medications you take vary by mistake, this then the type of diabetes and how well the causes the body to stop making insulin. With medicine controls you blood glucose levels. Type 2 diabetes the Type 1 diabetics must have insulin. Type 2 body does not respond may or may not include insulin and may just like it should to the be controlled with diet and exercise alone. insulin the pancreas is If you notice any of these changes notify making. Your body tells the pancreas that it needs to make more insulin since the your health care provider. The earlier • insulin that is already there is not working. -

Acute Renal Failure in Patients with Type 1 Diabetes Mellitus G
Postgrad Med J: first published as 10.1136/pgmj.70.821.192 on 1 March 1994. Downloaded from Postgrad Med J (1994) 70, 192- 194 C) The Fellowship of Postgraduate Medicine, 1994 Acute renal failure in patients with type 1 diabetes mellitus G. Woodrow, A.M. Brownjohn and J.H. Turney Renal Unit, Leeds General Infirmary, Great George Street, Leeds LSJ 3EX, UK Summary: Acute renal failure (ARF) is a serious condition which still carries a mortality of around 50%. People with diabetes may be at increased risk of developing ARF, either as a complication of diabetic ketoacidosis or hyperosmolar coma, increased incidence of cardiovascular disease, or due to increased susceptibility ofthe kidney to adverse effects in the presence ofunderlying diabetic renal disease. During the period 1956-1992, 1,661 cases of ARF have been treated at Leeds General Infirmary. Of these, we have identified 26 patients also having type 1 diabetes. ARF due to diabetic ketoacidosis is surprisingly uncommon (14 cases out of 23 patients whose notes were reviewed). All cases of ARF complicating ketoacidosis in the last decade have been associated with particularly severe illness requiring intensive care unit support, rather than otherwise 'uncomplicated' ketoacidosis. We discuss the conditions that may result in ARF in patients with diabetes and the particular difficulties that may be encountered in management. Introduction People with diabetes may be at increased risk of Results developing acute renal failure (ARF). Acute pre- copyright. renal failure may occur as a result ofthe severe fluid Of 23 patients with type 1 diabetes complicated by depletion associated with diabetic ketoacidosis and ARF, diabetic ketoacidosis was the main underly- non-ketotic hyperosmolar coma.