Ultrastructural Changes in Human Gall Bladder Epithelium in Cholelithiasis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Comparative Anatomy of the Lower Respiratory Tract of the Gray Short-Tailed Opossum (Monodelphis Domestica) and North American Opossum (Didelphis Virginiana)
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 12-2001 Comparative Anatomy of the Lower Respiratory Tract of the Gray Short-tailed Opossum (Monodelphis domestica) and North American Opossum (Didelphis virginiana) Lee Anne Cope University of Tennessee - Knoxville Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Animal Sciences Commons Recommended Citation Cope, Lee Anne, "Comparative Anatomy of the Lower Respiratory Tract of the Gray Short-tailed Opossum (Monodelphis domestica) and North American Opossum (Didelphis virginiana). " PhD diss., University of Tennessee, 2001. https://trace.tennessee.edu/utk_graddiss/2046 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Lee Anne Cope entitled "Comparative Anatomy of the Lower Respiratory Tract of the Gray Short-tailed Opossum (Monodelphis domestica) and North American Opossum (Didelphis virginiana)." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in Animal Science. Robert W. Henry, Major Professor We have read this dissertation and recommend its acceptance: Dr. R.B. Reed, Dr. C. Mendis-Handagama, Dr. J. Schumacher, Dr. S.E. Orosz Accepted for the Council: Carolyn R. -

Sphincter of Oddi: ERCP Plus Sphincterotomy – Yes Or No
Sphincter of Oddi: ERCP Plus Sphincterotomy – Yes or No Note: For debate purposes, the pro and con positions for patient management will be taken by the invited authors. However, actual decisions regarding patient care must involve discussion of the risks and benefits of each treatment considered. Case Presentation – Case developed by Ihab I. El Hajj, MD, MPH, Indiana University, Indianapolis, IN A 57-year-old Caucasian female with history of smoking and COPD, was in her usual state of health until two years ago, when she experienced recurrent “attacks” of right upper quadrant pain, nausea and occasional vomiting, suggestive of biliary colic. The patient was evaluated by her primary care physician and initial work- up, which included basic blood work, liver chemistries and transabdominal ultrasound, were negative. The patient responded partially to prn Zofran and omeprazole 40 mg once then twice daily. With the persistence of her symptoms, the patient was referred to a gastroenterologist. Esophagogastroduodenoscopy (EGD) with gastric biopsies revealed chronic inactive gastritis without Helicobacter pylori. HIDA scan suggested biliary dyskinesia with an ejection fraction of 22%. An elective laparoscopic cholecystectomy was performed. An intra- operative cholangiogram showed no filling defect in the common bile duct (CBD) and pathology demonstrated chronic cholecystitis with no gallstones. The patient was symptom-free for six months after surgery. She subsequently developed vague upper abdominal pain, intermittent nausea and irregular bowel movements. Labs, colonoscopy and repeat EGD were ASGE Leading Edge — Volume 4, No. 4 © American Society for Gastrointestinal Endoscopy normal. The patient was treated for suspected irritable bowel syndrome. She failed several medications including hyoscyamine, dicyclomine, amitriptyline, sucralfate, and GI cocktail. -
![Mft•] ~;;I~ [I) I~ T?L3 ·Ilr!F·S; [,J ~ M](https://docslib.b-cdn.net/cover/6471/mft-i-i-i-t-l3-%C2%B7ilr-f%C2%B7s-j-m-706471.webp)
Mft•] ~;;I~ [I) I~ T?L3 ·Ilr!F·S; [,J ~ M
Mft•] ~;;I~ [I) I~ t?l3 ·ilr!f·S; [,j ~ M Hepatobiliary Imaging Update Maggie Chester and Jerry Glowniak Veterans Affairs Medical Center and Oregon Health Sciences University, Portland, Oregon and the gallbladder ejection fraction (EF) after the injection This is the first article in a four-part series on interventional of cholecystokinin (CCK) (Kinevac®, Squibb Diagnostics, nuclear medicine. Upon completion, the nuclear medicine New Brunswick, NJ). A brief description of the hepatic ex technologist should be able to (1) list the advantages of using traction fraction (HEF) was given; the technique used quan interventional hepatic imaging, (2) identify the benefit in tifies hepatocyte function more accurately than does excretion calculating HEF, and (3) utilize the HEF calculation method when appropriate. half-time. Since publication of the previous article (5), the HEF has become more widely used as a measure of hepatocyte function, and nearly all the major nuclear medicine software vendors include programs for calculating the HEF. Scintigraphic assessment of hepatobiliary function began in In this article, we will describe new observations and meth the 1950s with the introduction of iodine-131 C31 1) Rose ods used in hepatobiliary imaging. The following topics will bengal (1). Due to the poor imaging characteristics of 1311, be discussed: ( 1) the use of morphine as an aid in the diagnosis numerous attempts were made to find a technetium-99m 99 of acute cholecystitis, (2) the rim sign in the diagnosis of acute ( mTc) labeled hepatobiliary agent (2). The most useful of cholecystitis, and (3) methods for calculating the HEF. the several 99mTc-labeled agents that were investigated were the iminodiacetic acid (IDA) analogs, which were introduced MORPHINE-AUGMENTED CHOLESCINTIGRAPHY in the mid 1970s (3). -

Progress Report Cholestasis and Lesions of the Biliary Tract in Chronic Pancreatitis
Gut: first published as 10.1136/gut.19.9.851 on 1 September 1978. Downloaded from Gut, 1978, 19, 851-857 Progress report Cholestasis and lesions of the biliary tract in chronic pancreatitis The occurrence of jaundice in the course of chronic pancreatitis has been recognised since the 19th century" 2. But in the early papers it is uncertain whether the cases were due to acute, acute relapsing, or to chronic pan- creatitis, or even to pancreatic cancer associated with pancreatitis or benign ampullary stenosis. With the introduction of endoscopic retrograde cholangiopancreato- graphy (ERCP), there has been a renewed interest in the biliary complica- tions of chronic pancreatitis (CP). However, papers published recently by endoscopists have generally neglected the cholangiographic aspect of the lesions and are less precise and less well documented than papers published just after the second world war, following the introduction of manometric cholangiography3-5. Furthermore, the description of obstructive jaundice due to chronic pancreatitis, classical 20 years ago, seems to have been forgotten until the recent papers. Radiological aspects of bile ducts in chronic pancreatitis http://gut.bmj.com/ If one limits the subject to primary diseases of the pancreas, particularly chronic calcifying pancreatitis (CCP)6, excluding chronic pancreatitis secondary to benign ampullary stenosis7, cancer obstructing the main pancreatic duct8 9 and acute relapsing pancreatitis secondary to gallstones'0 radiological aspect of the main bile duct" is type I the most.common on September 25, 2021 by guest. Protected copyright. choledocus (Figure). This description has been repeatedly confirmed'2"13. It is a long stenosis of the intra- or retropancreatic part of the main bile duct. -

Guideline for the Evaluation of Cholestatic Jaundice
CLINICAL GUIDELINES Guideline for the Evaluation of Cholestatic Jaundice in Infants: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition ÃRima Fawaz, yUlrich Baumann, zUdeme Ekong, §Bjo¨rn Fischler, jjNedim Hadzic, ôCara L. Mack, #Vale´rie A. McLin, ÃÃJean P. Molleston, yyEzequiel Neimark, zzVicky L. Ng, and §§Saul J. Karpen ABSTRACT Cholestatic jaundice in infancy affects approximately 1 in every 2500 term PREAMBLE infants and is infrequently recognized by primary providers in the setting of holestatic jaundice in infancy is an uncommon but poten- physiologic jaundice. Cholestatic jaundice is always pathologic and indicates tially serious problem that indicates hepatobiliary dysfunc- hepatobiliary dysfunction. Early detection by the primary care physician and tion.C Early detection of cholestatic jaundice by the primary care timely referrals to the pediatric gastroenterologist/hepatologist are important physician and timely, accurate diagnosis by the pediatric gastro- contributors to optimal treatment and prognosis. The most common causes of enterologist are important for successful treatment and an optimal cholestatic jaundice in the first months of life are biliary atresia (25%–40%) prognosis. The Cholestasis Guideline Committee consisted of 11 followed by an expanding list of monogenic disorders (25%), along with many members of 2 professional societies: the North American Society unknown or multifactorial (eg, parenteral nutrition-related) causes, each of for Gastroenterology, Hepatology and Nutrition, and the European which may have time-sensitive and distinct treatment plans. Thus, these Society for Gastroenterology, Hepatology and Nutrition. This guidelines can have an essential role for the evaluation of neonatal cholestasis committee has responded to a need in pediatrics and developed to optimize care. -

Biliary Tract Cancer*
Biliary Tract Cancer* What is Biliary Tract Cancer*? Let us answer some of your questions. * Cholangiocarcinoma (bile duct cancer) * Gallbladder cancer * Ampullary cancer ESMO Patient Guide Series based on the ESMO Clinical Practice Guidelines esmo.org Biliary tract cancer Biliary tract cancer* An ESMO guide for patients Patient information based on ESMO Clinical Practice Guidelines This guide has been prepared to help you, as well as your friends, family and caregivers, better understand biliary tract cancer and its treatment. It contains information on the causes of the disease and how it is diagnosed, up-to- date guidance on the types of treatments that may be available and any possible side effects of treatment. The medical information described in this document is based on the ESMO Clinical Practice Guideline for biliary tract cancer, which is designed to help clinicians with the diagnosis and management of biliary tract cancer. All ESMO Clinical Practice Guidelines are prepared and reviewed by leading experts using evidence gained from the latest clinical trials, research and expert opinion. The information included in this guide is not intended as a replacement for your doctor’s advice. Your doctor knows your full medical history and will help guide you regarding the best treatment for you. *Cholangiocarcinoma (bile duct cancer), gallbladder cancer and ampullary cancer. Words highlighted in colour are defined in the glossary at the end of the document. This guide has been developed and reviewed by: Representatives of the European -

Pancreaticobiliary Ductal Union Gut: First Published As 10.1136/Gut.31.10.1144 on 1 October 1990
1144 Gut, 1990, 31, 1144-1149 Pancreaticobiliary ductal union Gut: first published as 10.1136/gut.31.10.1144 on 1 October 1990. Downloaded from S P Misra, M Dwivedi Abstract TABLE ii Length ofthe common channel in normalsubjects in The main pancreatic duct and the common bile various series duct open into the second part of the duo- Authors Mean (mm) Range (mm) denum alone or after joining as a common Misra et al4 4-7 1-018-4 channel. A common channel of >15 mm (an Kimura et al5 4.6 2-10 anomalous pancreaticobiliary duct) is associ- Dowdy et al6 4-4 1-12 ated with congenital cystic dilatation of the common bile duct and carcinoma of the gall bladder. Even a long common channel channel of <3 mm, and 18% had a common (38 mm) is associated with a higher frequency channel of >3 mm. The rhean length was not of carcinoma of the gall bladder. Gail stones mentioned.2 In a necropsy study of 35 infants, smaller than the common channel and a long Miyano et al' noted that the average length of the common channel predispose to gail stone common channel was 1 3 mm. induced acute pancreatitis. Separate openings Kimura et al,' using cineradiography during for the two ductal systems predisposes to ERCP, have shown contractile motility of the development of gall stones and alcohol ductal wall extending well beyond the common induced chronic pancreatitis. The role of channel, towards the liver. The mean (SD) ductal union has also been investigated in length of the contractile segment was 20 5 primary sclerosing cholangitis and biliary (4 6) mm (range 14-31 mm). -
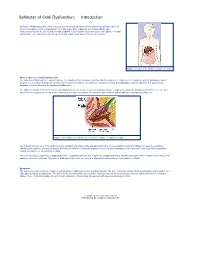
Sphincter of Oddi Dysfunction: Introduction
Sphincter of Oddi Dysfunction: Introduction Sphincter of Oddi dysfunction refers to structural or functional disorders involving the biliary sphincter that may result in impedance of bile and pancreatic juice flow. Up to 20% of patients with continued pain after cholecystectomy and 10–20% of patients with idiopathic recurrent pancreatitis may suffer from sphincter of Oddi dysfunction. This condition is more prevalent among middle-aged women for unclear reasons Figure 1. Location of the sphincter of Oddi in the body. What is Sphincter of Oddi Dysfunction? The sphincter of Oddi has three major functions: 1) regulation of bile and pancreatic flow into the duodenum, 2) diversion of hepatic bile into the gallbladder, and 3) the prevention of reflux of duodenal contents into the pancreaticobiliary tract. With the ingestion of a meal, the gallbladder contracts and there is a simultaneous decrease in the resistance in the sphincter of Oddi zone. The sphincter of Oddi consists of circular and longitudinal smooth muscle fibers surrounding a variable length of the distal bile and pancreatic duct. There are three discrete areas of muscle thickness, or mini sphincters: the sphincter papillae, the sphincter pancreaticus, and the sphincter choledochus (Figure 2). Figure 2. Mini sphincters, or discrete areas of muscle, comprise the sphincter of Oddi. The major physiologic role of the sphincter is the regulation of the flow of bile and pancreatic juice. Cholecystokinin (CCK) and nitrates decrease the resistance offered by the sphincter. Laboratory studies observing the effects of numerous peptides, hormones, and medications on the sphincter have suggested a multifactor control mechanism for the sphincter of Oddi. -

What Is the Normal Size of the Common Bile Duct?
DOI: https://doi.org/10.22516/25007440.136 Original articles What is the normal size of the common bile duct? Martín Alonso Gómez Zuleta, MD,1 Óscar Fernando Ruiz Morales, MD,2 William Otero Regino, MD.3 1 Internist and Gastroenterologist, Professor at the Abstract National University of Colombia. Gastroenterologist at National University of Colombia Hospital in Traditionally, the common bile duct (CBD) has been said to measure up to 6 mm in patients with gallbladders Bogotá, Colombia and up to 8 mm in cholecystectomized patients. However, these recommendations are based on very old 2 Internist and Gastroenterologist at Hospital Nacional studies performed with trans-abdominal ultrasound. Echoendoscopy has greater sensitivity and specificity for Universitario and Kennedy Hospital in Bogotá, Colombia evaluating the bile duct, but studies had not yet been done in our population to evaluate the normal size of 3 Internist and Gastroenterologist, Professor of the CBD by this method. Gastroenterology at the National University of Objective: The objective of this study was to evaluate the size of the CBD in patients with gallbladders and Colombia in Bogotá, Colombia patients without gallbladders. Materials and Methods: This is a prospective descriptive study of patients who underwent echoendoscopy ......................................... at the gastroenterology unit in the El Tunal hospital, Universidad Nacional de Colombia. Patients had been Received: 05-11-15 Accepted: 21-04-17 referred for diagnostic echoendoscopy to evaluate subepithelial lesions in the esophagus and/or stomach. Once the lesion had been evaluated and an echoendoscopic diagnosis had been established, the transducer was advanced to the second duodenal portion to perform bilio-pancreatic echoendoscopy. -

"Neural Interactions Between the Small Intestine and the Sphincter of Oddi" and "Sustainable Development for Economic, Social, and Environmental Health"
Bridgewater Review Volume 19 | Issue 1 Article 11 Jun-2000 Report From CART: "Neural Interactions Between the Small Intestine and the Sphincter of Oddi" And "Sustainable Development for Economic, Social, and Environmental Health" Recommended Citation (2000). Report From CART: "Neural Interactions Between the Small Intestine and the Sphincter of Oddi" And "Sustainable Development for Economic, Social, and Environmental Health". Bridgewater Review, 19(1), 25-26. Available at: http://vc.bridgew.edu/br_rev/vol19/iss1/11 This item is available as part of Virtual Commons, the open-access institutional repository of Bridgewater State University, Bridgewater, Massachusetts. duodenal neurons sending nerve fibers to the sphincter ofOddi. We deter CENTER FOR mined that these neurons synthesize excitatory neurotransmitters, which cause the sphincter muscle to contract. THE ADVANCEMENT We performed additional electro physiological studies to further exam OF RESEARCH ine this mechanism. These studies involved recording electrical activity from target neurons located within the AND TEACHING sphincter ofOddi while stimulating axons passing into the sphincter from CART grants enable faculty and results in over 600,000 cholecystec the duodenum. We demonstrated that librarians to pursue research projects. tomies (surgical removals ofthe the duodenal neurons sending projec "Neural Interactions Between the Small gallbladder) each year. tions to the sphincter ofOddi are capa Intestine and the Sphincter ofOddi" How does the sphincter ofOddi ble ofelectrically activating neurons and "Sustainable Development: The know when to open and when to close? located within the sphincter. Activation Search for Economic, Social, and Although we know that the nervous ofneurons in the sphincter ofOddi by Environmental Health" are among the system is involved, the exact mecha neurons in the duodenum is likely to projects which were recently awarded nisms ofits regulation are not entirely increase the contraction ofthis muscle, CART grants. -

Expression of Specific Hepatocyte and Cholangiocyte Transcription Factors
Laboratory Investigation (2008) 88, 865–872 & 2008 USCAP, Inc All rights reserved 0023-6837/08 $30.00 Expression of specific hepatocyte and cholangiocyte transcription factors in human liver disease and embryonic development Pallavi B Limaye, Gabriela Alarco´n, Andrew L Walls, Michael A Nalesnik, George K Michalopoulos, Anthony J Demetris and Erin R Ochoa Transcription factors are major determinants of cell-specific gene expression in all cell types. Studies in rodent liver have shown that alterations in transcription factor expression determine lineage specification during fetal liver development and signify transdifferentiation of cells of the biliary compartment into ‘oval’ cells and eventually hepatocytes in adult liver. We examined the cellular localization of hepatocyte- or BEC-associated transcription factors in human fetal and adult liver and in diseases in which transdifferentiation between hepatocytes and biliary cells may play a role. In the normal adult human liver, hepatocyte nuclear factor (HNF)4a and HNF6 appeared exclusively in hepatocytes; HNF1b, HNF3a, and HNF3b were observed only in BEC. During fetal development both BEC and hepatocytes expressed HNF3a, HNF3b, and HNF6. HNF1a was expressed only in fetal hepatocytes. We further examined expression of transcription factors in massive hepatic necrosis and in specific types of chronic liver disease. Hepatocyte-associated transcription factors HNF4a and HNF6 also appeared in BEC in massive hepatic necrosis and chronic hepatitis C virus infection. Similarly, HNF3b that is expressed only in BEC in normal adult liver was also observed in hepatocytes in primary biliary cirrhosis and chronic biliary obstruction. These data mimic previous findings in rodents in which hepatocyte-associated transcription factors appear in biliary cells prior to emergence of oval cells, which function as progenitor cells for hepatocytes when the regenerative capacity of the latter is compromised. -

Chronic Pancreatitis: Introduction
Chronic Pancreatitis: Introduction Authors: Anthony N. Kalloo, MD; Lynn Norwitz, BS; Charles J. Yeo, MD Chronic pancreatitis is a relatively rare disorder occurring in about 20 per 100,000 population. The disease is progressive with persistent inflammation leading to damage and/or destruction of the pancreas . Endocrine and exocrine functional impairment results from the irreversible pancreatic injury. The pancreas is located deep in the retroperitoneal space of the upper part of the abdomen (Figure 1). It is almost completely covered by the stomach and duodenum . This elongated gland (12–20 cm in the adult) has a lobe-like structure. Variation in shape and exact body location is common. In most people, the larger part of the gland's head is located to the right of the spine or directly over the spinal column and extends to the spleen . The pancreas has both exocrine and endocrine functions. In its exocrine capacity, the acinar cells produce digestive juices, which are secreted into the intestine and are essential in the breakdown and metabolism of proteins, fats and carbohydrates. In its endocrine function capacity, the pancreas also produces insulin and glucagon , which are secreted into the blood to regulate glucose levels. Figure 1. Location of the pancreas in the body. What is Chronic Pancreatitis? Chronic pancreatitis is characterized by inflammatory changes of the pancreas involving some or all of the following: fibrosis, calcification, pancreatic ductal inflammation, and pancreatic stone formation (Figure 2). Although autopsies indicate that there is a 0.5–5% incidence of pancreatitis, the true prevalence is unknown. In recent years, there have been several attempts to classify chronic pancreatitis, but these have met with difficulty for several reasons.