Sphincter of Oddi Function and Risk Factors for Dysfunction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Comparative Anatomy of the Lower Respiratory Tract of the Gray Short-Tailed Opossum (Monodelphis Domestica) and North American Opossum (Didelphis Virginiana)
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 12-2001 Comparative Anatomy of the Lower Respiratory Tract of the Gray Short-tailed Opossum (Monodelphis domestica) and North American Opossum (Didelphis virginiana) Lee Anne Cope University of Tennessee - Knoxville Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Animal Sciences Commons Recommended Citation Cope, Lee Anne, "Comparative Anatomy of the Lower Respiratory Tract of the Gray Short-tailed Opossum (Monodelphis domestica) and North American Opossum (Didelphis virginiana). " PhD diss., University of Tennessee, 2001. https://trace.tennessee.edu/utk_graddiss/2046 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Lee Anne Cope entitled "Comparative Anatomy of the Lower Respiratory Tract of the Gray Short-tailed Opossum (Monodelphis domestica) and North American Opossum (Didelphis virginiana)." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in Animal Science. Robert W. Henry, Major Professor We have read this dissertation and recommend its acceptance: Dr. R.B. Reed, Dr. C. Mendis-Handagama, Dr. J. Schumacher, Dr. S.E. Orosz Accepted for the Council: Carolyn R. -

Sphincter of Oddi: ERCP Plus Sphincterotomy – Yes Or No
Sphincter of Oddi: ERCP Plus Sphincterotomy – Yes or No Note: For debate purposes, the pro and con positions for patient management will be taken by the invited authors. However, actual decisions regarding patient care must involve discussion of the risks and benefits of each treatment considered. Case Presentation – Case developed by Ihab I. El Hajj, MD, MPH, Indiana University, Indianapolis, IN A 57-year-old Caucasian female with history of smoking and COPD, was in her usual state of health until two years ago, when she experienced recurrent “attacks” of right upper quadrant pain, nausea and occasional vomiting, suggestive of biliary colic. The patient was evaluated by her primary care physician and initial work- up, which included basic blood work, liver chemistries and transabdominal ultrasound, were negative. The patient responded partially to prn Zofran and omeprazole 40 mg once then twice daily. With the persistence of her symptoms, the patient was referred to a gastroenterologist. Esophagogastroduodenoscopy (EGD) with gastric biopsies revealed chronic inactive gastritis without Helicobacter pylori. HIDA scan suggested biliary dyskinesia with an ejection fraction of 22%. An elective laparoscopic cholecystectomy was performed. An intra- operative cholangiogram showed no filling defect in the common bile duct (CBD) and pathology demonstrated chronic cholecystitis with no gallstones. The patient was symptom-free for six months after surgery. She subsequently developed vague upper abdominal pain, intermittent nausea and irregular bowel movements. Labs, colonoscopy and repeat EGD were ASGE Leading Edge — Volume 4, No. 4 © American Society for Gastrointestinal Endoscopy normal. The patient was treated for suspected irritable bowel syndrome. She failed several medications including hyoscyamine, dicyclomine, amitriptyline, sucralfate, and GI cocktail. -
![Mft•] ~;;I~ [I) I~ T?L3 ·Ilr!F·S; [,J ~ M](https://docslib.b-cdn.net/cover/6471/mft-i-i-i-t-l3-%C2%B7ilr-f%C2%B7s-j-m-706471.webp)
Mft•] ~;;I~ [I) I~ T?L3 ·Ilr!F·S; [,J ~ M
Mft•] ~;;I~ [I) I~ t?l3 ·ilr!f·S; [,j ~ M Hepatobiliary Imaging Update Maggie Chester and Jerry Glowniak Veterans Affairs Medical Center and Oregon Health Sciences University, Portland, Oregon and the gallbladder ejection fraction (EF) after the injection This is the first article in a four-part series on interventional of cholecystokinin (CCK) (Kinevac®, Squibb Diagnostics, nuclear medicine. Upon completion, the nuclear medicine New Brunswick, NJ). A brief description of the hepatic ex technologist should be able to (1) list the advantages of using traction fraction (HEF) was given; the technique used quan interventional hepatic imaging, (2) identify the benefit in tifies hepatocyte function more accurately than does excretion calculating HEF, and (3) utilize the HEF calculation method when appropriate. half-time. Since publication of the previous article (5), the HEF has become more widely used as a measure of hepatocyte function, and nearly all the major nuclear medicine software vendors include programs for calculating the HEF. Scintigraphic assessment of hepatobiliary function began in In this article, we will describe new observations and meth the 1950s with the introduction of iodine-131 C31 1) Rose ods used in hepatobiliary imaging. The following topics will bengal (1). Due to the poor imaging characteristics of 1311, be discussed: ( 1) the use of morphine as an aid in the diagnosis numerous attempts were made to find a technetium-99m 99 of acute cholecystitis, (2) the rim sign in the diagnosis of acute ( mTc) labeled hepatobiliary agent (2). The most useful of cholecystitis, and (3) methods for calculating the HEF. the several 99mTc-labeled agents that were investigated were the iminodiacetic acid (IDA) analogs, which were introduced MORPHINE-AUGMENTED CHOLESCINTIGRAPHY in the mid 1970s (3). -
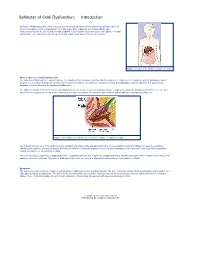
Sphincter of Oddi Dysfunction: Introduction
Sphincter of Oddi Dysfunction: Introduction Sphincter of Oddi dysfunction refers to structural or functional disorders involving the biliary sphincter that may result in impedance of bile and pancreatic juice flow. Up to 20% of patients with continued pain after cholecystectomy and 10–20% of patients with idiopathic recurrent pancreatitis may suffer from sphincter of Oddi dysfunction. This condition is more prevalent among middle-aged women for unclear reasons Figure 1. Location of the sphincter of Oddi in the body. What is Sphincter of Oddi Dysfunction? The sphincter of Oddi has three major functions: 1) regulation of bile and pancreatic flow into the duodenum, 2) diversion of hepatic bile into the gallbladder, and 3) the prevention of reflux of duodenal contents into the pancreaticobiliary tract. With the ingestion of a meal, the gallbladder contracts and there is a simultaneous decrease in the resistance in the sphincter of Oddi zone. The sphincter of Oddi consists of circular and longitudinal smooth muscle fibers surrounding a variable length of the distal bile and pancreatic duct. There are three discrete areas of muscle thickness, or mini sphincters: the sphincter papillae, the sphincter pancreaticus, and the sphincter choledochus (Figure 2). Figure 2. Mini sphincters, or discrete areas of muscle, comprise the sphincter of Oddi. The major physiologic role of the sphincter is the regulation of the flow of bile and pancreatic juice. Cholecystokinin (CCK) and nitrates decrease the resistance offered by the sphincter. Laboratory studies observing the effects of numerous peptides, hormones, and medications on the sphincter have suggested a multifactor control mechanism for the sphincter of Oddi. -

"Neural Interactions Between the Small Intestine and the Sphincter of Oddi" and "Sustainable Development for Economic, Social, and Environmental Health"
Bridgewater Review Volume 19 | Issue 1 Article 11 Jun-2000 Report From CART: "Neural Interactions Between the Small Intestine and the Sphincter of Oddi" And "Sustainable Development for Economic, Social, and Environmental Health" Recommended Citation (2000). Report From CART: "Neural Interactions Between the Small Intestine and the Sphincter of Oddi" And "Sustainable Development for Economic, Social, and Environmental Health". Bridgewater Review, 19(1), 25-26. Available at: http://vc.bridgew.edu/br_rev/vol19/iss1/11 This item is available as part of Virtual Commons, the open-access institutional repository of Bridgewater State University, Bridgewater, Massachusetts. duodenal neurons sending nerve fibers to the sphincter ofOddi. We deter CENTER FOR mined that these neurons synthesize excitatory neurotransmitters, which cause the sphincter muscle to contract. THE ADVANCEMENT We performed additional electro physiological studies to further exam OF RESEARCH ine this mechanism. These studies involved recording electrical activity from target neurons located within the AND TEACHING sphincter ofOddi while stimulating axons passing into the sphincter from CART grants enable faculty and results in over 600,000 cholecystec the duodenum. We demonstrated that librarians to pursue research projects. tomies (surgical removals ofthe the duodenal neurons sending projec "Neural Interactions Between the Small gallbladder) each year. tions to the sphincter ofOddi are capa Intestine and the Sphincter ofOddi" How does the sphincter ofOddi ble ofelectrically activating neurons and "Sustainable Development: The know when to open and when to close? located within the sphincter. Activation Search for Economic, Social, and Although we know that the nervous ofneurons in the sphincter ofOddi by Environmental Health" are among the system is involved, the exact mecha neurons in the duodenum is likely to projects which were recently awarded nisms ofits regulation are not entirely increase the contraction ofthis muscle, CART grants. -

Division of Nuclear Medicine Procedure / Protocol University
Division of Nuclear Medicine Procedure / Protocol University Hospital and The American Center ________________________________________________________________________________________________ HEPATOBILIARY IMAGING CPT CODE: 78223 UPDATED: JULY 2017 ________________________________________________________________________________________________ Indications: • Diagnosis of acute cholecystitis (both calculus and acalculous disease) • Determination of patency of common bile duct when ultrasound examination not diagnostic (e.g., very early obstruction) • Evaluation of biliary dyskinesia (gallbladder ejection fraction [GBEF] test) for chronic cholecystitis • Identification of biliary leaks • Differentiation of biliary atresia from neonatal hepatitis • Evaluation of presence, or absence of, spleen (with T-99m SC Liver Scan). • Suspected sphincter of Oddi dysfunction or partial biliary obstruction due to stones or stricture. Patient Preps: Acute Cholecystitis See Acute Cholecystitis algorithm below, Appendix 2 No Pre-Treatment: • Fasting for a minimum of 2 hours, but preferably 4 hours before this test. • Parental alimentation (ex. TPN) is allowable and considered to be a fasting state. Pre-Treatment: • Record any recent hydromorphone (Dilaudid), fentanyl, or morphine used in last 12 hours ; refer to morphine use section below and opioid table in Appendix 1. • In patients who have fasted for greater than 24 hours, on parenteral nutrition, or with a history of alcoholic liver disease, Sincalide (Kinevac) should be administered prior to tracer injection. Sincalide administration of 0.02 microgram/kg is to be over 30 min. Mebrofenin injection can be started 10-60 min after Sincalide infusion is completed. On-call cases of fasting state for pre- treatment sincalide administration should be determined by the technologist with the patient’s nurse (** If there is any question or lack of information of fasting state, sincalide pre-treatment should be administered prior to imaging to clear the gallbladder. -

2015 AACA Annual Meeting Program
June 9 – 12, 2015 | Henderson, Nevada President’s Report June 9-12, 2015 Green Valley Ranch Resort & Casino Henderson, NV Another year has quickly passed and I have been asked to summarize achievements/threats to the Association for our meeting program booklet. Much of this will be recanted in my introductory message on the opening day of the meeting in Henderson. As President, I am representing Council in recognizing the work of those individuals not already recognized in our standing committee reports that you will find in this program. One of our most active ad hoc committees has been the one looking into creating an endowment for the association through member and vendor sponsorships. Our past president, Anne Agur, has chaired this committee and deserves accolades for having the committee work hard and produce the materials you have either already seen, or will be introduced to in Henderson. The format was based on that used by many clinical organizations. It allows support at many different levels, the financial income from which is being invested for student awards and travel stipends. Our ambitious 5 year goal is $100,000. I hope that you will join me in thinking seriously about supporting this initiative - at whichever level you feel comfortable with. Every dollar goes to the endowment. In October, Council ratified the creation of our new standing committee - Brand Promotion and Outreach. This committee was formed by fusing the two ad hoc committees struck by Anne Agur when she was President. Last year our new branding was highly visible in Orlando and we want to use this momentum to continue raising the profile of the Association at many different types of events within and outside North America. -

Diagnostics of Halitosis Complaints by a Multidisciplinary Team
JOP. J Pancreas (Online) 2014 Nov 28; 15(6): 552-560 REVIEW ARTICLE Chronic Metabolic Acidosis Destroys Pancreas Peter Melamed and Felix Melamed Biotherapy Clinic of San Francisco, USA ABSTRACT One primary reason for the current epidemic of digestive disorders might be chronic metabolic acidosis, which is extremely common in the modern population. Chronic metabolic acidosis primarily affects two alkaline digestive glands, the liver, and the pancreas, which produce alkaline bile and pancreatic juice with a large amount of bicarbonate. Even small acidic alterations in the bile and pancreatic juice pH can lead to serious biochemical/biomechanical changes. The pancreatic digestive enzymes require an alkaline milieu for proper function, antimicrobial activity, which can lead to intestinal dysbiosis. Lowering the pH of the pancreatic juice can cause premature activation of the proteasesand lowering inside the the pH pancreas disables withtheir theactivity. potential It can development be the primary of pancreatitis. cause of indigestion. Acidification of the pancreatic juice decreases its Aggressive mixture of the acidic bile and the pancreatic juice can cause erratic contractions of the duodenum’s walls and subsequent bile The acidification of bile causes precipitation of the bile acids, which irritate the entire biliary system and create bile stone formation. effective and safe treatment for enhancing the exocrine pancreatic function. Restoring normal acid-base homeostasis can be a useful tool forreflux pathophysiological into the stomach therapeutic and the esophagus. approaches Normal for various exocrine gastrointestinal pancreatic function disorders. is Therethe core is strongof proper research digestion. and practical Currently, evidence there is that no - restoring the HCO3 capacity in the blood can improve digestion. -

Ultrastructural Changes in Human Gall Bladder Epithelium in Cholelithiasis
Dec QMJ VOL.8 No.12 Ultrastructural Changes In Human Gall Bladder Epithelium In Cholelithiasis Khalida I. Shaya*, Muna Zuhair**and Modhar S. Al-Omary*** اﻟﺨﻼﺻﺔ ﺗﻜﻮﯾﻦ اﻟﺤﺼﻮات اﻟﺼﻔﺮاوﯾﺔ ﯾﻌﺮف ﻋﻠﻰ اﻧﮫ وﺟﻮد ﺣﺼﻰ ﺿﻤﻦ ﺗﺠﻮﯾﻒ اﻛﯿﺎس اﻟﻤﺮارة او ﻓﻲ اﻟﺸﺠﯿﺮات اﻟﺼﻔﺮاوﯾﺔ اﻟﺨﺎرج ﻛﺒﺪﯾﺔ. اﻟﮭﺪف ﻣﻦ ھﺬه اﻟﺪراﺳﺔ ھﻮ اﻟﺘﻌﺮف ﻋﻠﻰ اﻟﺘﻐﯿﯿﺮات اﻟﻨﺴﯿﺠﯿﺔ اﻟﺪﻗﯿﻘﺔ ﻓﻲ اﻟﻨﺴﯿﺞ اﻟﻄﻼﺋﻲ ﻻﻛﯿﺎس اﻟﻤﺮارة ﻓﻲ ﺣﺎﻟﺔ ﺗﻜﻮﯾﻦ اﻟﺤﺼﻮات اﻟﺼﻔﺮاوﯾﺔ . ﻧﻤﺎذج اﻛﯿﺎس اﻟﻤﺮارة ﺟﻤﻌﺖ ﻣﻦ ﻣﺮﺿﻰ اﺟﺮﯾﺖ ﻟﮭﻢ ﻋﻤﻠﯿﺔ اﺳﺘﺌﺼﺎل اﻛﯿﺎس اﻟﻤﺮارة. اﻟﻨﻤﺎذج اﻟﺪﻗﯿﻘﺔ ﺣﻀﺮت و ﻋﻮﻣﻠﺖ ﻟﺪراﺳﺔ اﻟﺘﺮﻛﯿﺐ اﻟﻨﺴﯿﺠﻲ اﻟﺪﻗﯿﻖ ﻟﻠﻨﺴﯿﺞ اﻟﻄﻼﺋﻲ ﻻﻛﯿﺎس اﻟﻤﺮارة . اﻟﺘﻐﯿﯿﺮات اﻟﻨﺴﯿﺠﯿﺔ اﻟﻈﺎھﺮة ﻓﻲ اﻟﻤﻘﺎﻃﻊ اﻟﺸﺒﮫ رﻗﯿﻘﺔ ﻟﺼﺒﻐﺔ اﻟﻤﺜﯿﻞ اﻻزرق اﻇﮭﺮت وﺟﻮد ﺗﻤﺰﻗﺎت ﻣﻊ ﻋﺪم ﺗﻤﺎﺳﻚ ﻓﻲ اﻟﻨﺴﯿﺞ اﻟﻄﻼﺋﻲ ﻟﻠﻤﺮارة ﻣﺘﺮاﻓﻘﺎ ﻣﻊ ﻇﮭﻮر ﺗﺠﺎوﯾﻒ روﻛﺘﺎﻧﺴﻜﻲ اﺷﻮف ﻣﻊ وﺟﻮد ﺗﻀﺨﻢ وﻋﺪم اﻧﺘﻈﺎم اﻟﺘﻨﺎﺳﻖ ﻓﻲ اﻟﺨﻼﯾﺎ اﻟﻄﻼﺋﯿﺔ ﻣﻊ ﺣﺆول ﻓﻲ اﻟﻐﺪد اﻟﻤﺨﺎﻃﯿﺔ ﻓﻲ اﻟﺼﻔﯿﺤﺔ اﻟﺮﻗﯿﻘﺔ ﻻﻛﯿﺎس اﻟﻤﺮارة. ﻓﺤﺺ اﻟﺘﺮﻛﯿﺐ اﻟﻨﺴﯿﺠﻲ اﻟﺪﻗﯿﻖ اﻇﮭﺮ وﺟﻮد ﺳﺤﺞ ﻓﻲ اﻟﺰﻏﯿﺒﺎت اﻟﺪﻗﯿﻘﺔ ﻣﻊ ﺗﺤﻄﻢ ﻟﺒﯿﻮت اﻟﻄﺎﻗﺔ و ﺗﻮﺳﻊ ﻓﻲ اﻟﻔﺮاﻏﺎت ﺑﯿﻦ اﻟﺨﻠﻮﯾﺔ ﻣﺘﺰاﻣﻨﺎ ﻣﻊ ﻇﮭﻮر اﻟﻔﺠﻮات ﻓﻲ داﺧﻞ اﻟﺨﻼﯾﺎ اﻟﻄﻼﺋﯿﺔ ﻣﻊ وﺟﻮد ﻋﺪم اﻧﺘﻈﺎم و اﻧﻔﺘﺎق ﻓﻲ ﻣﺤﯿﻂ اﻟﺨﻼﯾﺎ. اﻟﺨﻼﯾﺎ اﻟﻄﻼﺋﯿﺔ اﺣﺘﻮت ﻋﻠﻰ ﻗﻄﯿﺮات ﻣﺨﺎﻃﯿﺔ، ﺷﻮھﺪ ﺑﻌﻀﮭﺎ ﻣﻔﺮزا اﻟﻰ ﺧﺎرج اﻟﺨﻠﯿﺔ . ﺣﺼﻰ اﻟﻤﺮارة ﯾﺮاﻓﻘﮭﺎ ﺗﻐﯿﯿﺮات ﻧﺴﯿﺠﯿﺔ اﺳﺎﺳﯿﺔ ﻓﻲ اﻻﻧﺴﺠﺔ اﻟﻄﻼﺋﯿﺔ ﻛﻤﺎ ﻇﮭﺮت ﻓﻲ اﻟﻤﺠﮭﺮﯾﻦ اﻟﻀﻮﺋﻲ و اﻻﻟﻜﺘﺮوﻧﻲ . ﻣﻔﺘﺎح اﻟﻜﻠﻤﺎت: اﻛﯿﺎس اﻟﻤﺮارة، ﺗﻜﻮﯾﻦ اﻟﺤﺼﻮات اﻟﺼﻔﺮاوﯾﺔ، اﻟﺘﻐﯿﺮات اﻟﻨﺴﯿﺠﯿﺔ اﻟﺪﻗﯿﻘﺔ. Abstract Cholelithiasis is defined as the presence of stones within the lumen of the gall bladder or in the extrahepatic biliary tree. The goal of this study was to identify the ultrastructural ultrations of gall bladder epithelium in cholelithiesis. Gallbladder specimens were collected from patients who underwent cholectstectomy. Minute specimens were also fixed and processed to evaluate the fine structures of the gall bladder epithelium. -

Gallbladder and Sphincter of Oddi Disorders Peter B
Gastroenterology 2016;150:1420–1429 Gallbladder and Sphincter of Oddi Disorders Peter B. Cotton,1 Grace H. Elta,2 C. Ross Carter,3 Pankaj Jay Pasricha,4 Enrico S. Corazziari5 1Medical University of South Carolina, Charleston, South Carolina; 2University of Michigan, Ann Arbor, Michigan; 3Glasgow Royal Infirmary, Glasgow, Scotland; 4Johns Hopkins School of Medicine, Baltimore, Maryland; and 5Universita La Sapienza, Rome, Italy The concept that motor disorders of the gallbladder, cystic duct, and sphincter of Oddi can cause painful syndromes is E1. Diagnostic Criteria for Biliary Pain attractive and popular, at least in the United States. How- Pain located in the epigastrium and/or right upper ever, the results of commonly performed ablative treat- quadrant and all of the following: ments (eg, cholecystectomy and sphincterotomy) are not uniformly good. The predictive value of tests that are often 1. Builds up to a steady level and lasting 30 minutes GALLBLADDER AND SOD used to diagnose dysfunction (eg, dynamic gallbladder or longer scintigraphy and sphincter manometry) is controversial. Evaluation and management of these patients is made 2. Occurring at different intervals (not daily) difficult by the fluctuating symptoms and the placebo effect 3. Severe enough to interrupt daily activities or lead of invasive interventions. A recent stringent study has to an emergency department visit shown that sphincterotomy is no better than sham treat- ment in patients with post-cholecystectomy pain and little 4. Not significantly (<20%) related to bowel or no objective abnormalities on investigation, so that the movements old concept of sphincter of Oddi dysfunction type III is dis- 5. Not significantly (<20%) relieved by postural carded. -

Historia Natural Del Cáncer De Páncreas En El Departamento De Salud De Elda”
UNIVERSIDAD MIGUEL HERNÁNDEZ “Historia Natural del Cáncer de Páncreas en el Departamento de Salud de Elda” Departamento de Medicina Clínica TESIS DOCTORAL Sonia Alonso Hernández Junio de 2017 1 D. Javier Fernández Sánchez, Director del Departamento de Medicina Clínica de la Universidad Miguel Hernández AUTORIZA: La presentación y defensa como Tesis Doctoral del trabajo “Historia Natural del Cáncer de páncreas en el departamento de Salud del Elda” presentado por Dña. Sonia Alonso Hernández bajo la dirección del Dr. Rafael Durán García y del Dr. Vicente Francisco Gil Guillén. Lo que firmo en San Juan de Alicante a 4 de Julio de 2017 Prof. D. Javier Fernández Sánchez Director Dpto. Medicina Clínica DEPARTAMENTO DE MEDICINA CLINICA Campus de San Juan. Ctra. de Valencia (N·332), Km. 87 03550 San Juan de Alicante Telf.: 96 5919449 – Fax: 96 5919450 c.electrónico: [email protected] 2 Don VICENTE FRANCISCO GIL GUILLÉN y Don RAFAEL DURÁN GARCÍA como Directores de la Tesis Doctoral CERTIFICAN Que el trabajo “Historia Natural del Cáncer de Páncreas en el Departamento de Salud de Elda” realizado por Dña. Sonia Alonso Hernández ha sido llevado a cabo bajo nuestra dirección y se encuentra en condiciones de ser leído y defendido como Tesis Doctoral en la Universidad Miguel Hernández. Lo que firmamos para los oportunos efectos en San Juan de Alicante a 29 de Junio de 2017 Fdo. Dr. D. Vicente Francisco Gil Guillén Director Tesis Doctoral Fdo. Dr. D. Rafael Durán García Director Tesis Doctoral 3 UNIVERSIDAD MIGUEL HERNÁNDEZ FACULTAD DE MEDICINA Departamento de Medicina Clínica HISTORIA NATURAL DEL CÁNCER DE PÁNCREAS EN EL DEPARTAMENTO DE SALUD DE ELDA. -

Sphincter of Oddi Dysfunction, Manometry, Oddi Disorder
Journal of Medicine and Life Vol. 1, No.2, April-June 2008 The functional sphincter of Oddi disorder Corina Pop, Adina Purcăreanu, Monica Purcărea, Dan Andronescu Department Of Gastroenterology And Internal Medicine University Hospital Bucharest Correspondence to: Dan Andronescu, M.D., Ph.D., Emergency Universitary Hospital Bucharest, Splaiul Independentei 169, Bucharest 050098, Romania Abstract The sphincter of Oddi disorder (SOD) has been a controversial subject for many years, about which a lot has been written. However, new findings mainly using Endoscopic Retrograde Cholangiopancreatography (ERCP) and sphincter of Oddi manometry (SOM) demonstrate the fact of this diagnostic. SOD is just a part of a larger pathology, the functional gastrointestinal disorders, which have been reconsidered as an important part of gastrointestinal diseases. For a better understanding, the American Gastroenterology Association Institute created a new classification of The Functional Gastrointestinal Disorders in 2006, Rome III Classification, in which the SOD is grouped in the functional biliary disorders (category E). The term SOD is used to define manometric abnormalities in patients who have signs and symptoms consistent with a biliary or pancreatic ductal origin. Based on the pathogenic mechanism and manometry findings, the SOD is separated into two groups: a group characterized by a stenotic pattern (anatomical abnormality) and a second group with a dyskinetic pattern (functional abnormality). The purpose of this article is to construct a short presentation of the main aspects regarding functional SOD (E2 and E3 after Rome III Classification). Keywords: sphincter of Oddi, functional disorder, sphincter of Oddi dysfunction, manometry, Oddi disorder Introduction Functional biliary disorders are common gastrointestinal diseases seen in general practice and by the gastroenterologist.