The Origin of Angiosperms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

JUDD W.S. Et. Al. (2002) Plant Systematics: a Phylogenetic Approach. Chapter 7. an Overview of Green
UNCORRECTED PAGE PROOFS An Overview of Green Plant Phylogeny he word plant is commonly used to refer to any auto- trophic eukaryotic organism capable of converting light energy into chemical energy via the process of photosynthe- sis. More specifically, these organisms produce carbohydrates from carbon dioxide and water in the presence of chlorophyll inside of organelles called chloroplasts. Sometimes the term plant is extended to include autotrophic prokaryotic forms, especially the (eu)bacterial lineage known as the cyanobacteria (or blue- green algae). Many traditional botany textbooks even include the fungi, which differ dramatically in being heterotrophic eukaryotic organisms that enzymatically break down living or dead organic material and then absorb the simpler products. Fungi appear to be more closely related to animals, another lineage of heterotrophs characterized by eating other organisms and digesting them inter- nally. In this chapter we first briefly discuss the origin and evolution of several separately evolved plant lineages, both to acquaint you with these important branches of the tree of life and to help put the green plant lineage in broad phylogenetic perspective. We then focus attention on the evolution of green plants, emphasizing sev- eral critical transitions. Specifically, we concentrate on the origins of land plants (embryophytes), of vascular plants (tracheophytes), of 1 UNCORRECTED PAGE PROOFS 2 CHAPTER SEVEN seed plants (spermatophytes), and of flowering plants dons.” In some cases it is possible to abandon such (angiosperms). names entirely, but in others it is tempting to retain Although knowledge of fossil plants is critical to a them, either as common names for certain forms of orga- deep understanding of each of these shifts and some key nization (e.g., the “bryophytic” life cycle), or to refer to a fossils are mentioned, much of our discussion focuses on clade (e.g., applying “gymnosperms” to a hypothesized extant groups. -

Ecological Sorting of Vascular Plant Classes During the Paleozoic Evolutionary Radiation
i1 Ecological Sorting of Vascular Plant Classes During the Paleozoic Evolutionary Radiation William A. DiMichele, William E. Stein, and Richard M. Bateman DiMichele, W.A., Stein, W.E., and Bateman, R.M. 2001. Ecological sorting of vascular plant classes during the Paleozoic evolutionary radiation. In: W.D. Allmon and D.J. Bottjer, eds. Evolutionary Paleoecology: The Ecological Context of Macroevolutionary Change. Columbia University Press, New York. pp. 285-335 THE DISTINCTIVE BODY PLANS of vascular plants (lycopsids, ferns, sphenopsids, seed plants), corresponding roughly to traditional Linnean classes, originated in a radiation that began in the late Middle Devonian and ended in the Early Carboniferous. This relatively brief radiation followed a long period in the Silurian and Early Devonian during wrhich morphological complexity accrued slowly and preceded evolutionary diversifications con- fined within major body-plan themes during the Carboniferous. During the Middle Devonian-Early Carboniferous morphological radiation, the major class-level clades also became differentiated ecologically: Lycopsids were cen- tered in wetlands, seed plants in terra firma environments, sphenopsids in aggradational habitats, and ferns in disturbed environments. The strong con- gruence of phylogenetic pattern, morphological differentiation, and clade- level ecological distributions characterizes plant ecological and evolutionary dynamics throughout much of the late Paleozoic. In this study, we explore the phylogenetic relationships and realized ecomorphospace of reconstructed whole plants (or composite whole plants), representing each of the major body-plan clades, and examine the degree of overlap of these patterns with each other and with patterns of environmental distribution. We conclude that 285 286 EVOLUTIONARY PALEOECOLOGY ecological incumbency was a major factor circumscribing and channeling the course of early diversification events: events that profoundly affected the structure and composition of modern plant communities. -

Fundamentals of Palaeobotany Fundamentals of Palaeobotany
Fundamentals of Palaeobotany Fundamentals of Palaeobotany cuGU .叮 v FimditLU'φL-EjAA ρummmm 吋 eαymGfr 伊拉ddd仇側向iep M d、 況 O C O W Illustrations by the author uc削 ∞叩N Nn凹創 刊,叫MH h 咀 可 白 a aEE-- EEA First published in 1987 by Chapman αndHallLtd 11 New Fetter Lane, London EC4P 4EE Published in the USA by Chα~pman and H all 29 West 35th Street: New Yo地 NY 10001 。 1987 S. V. M秒len Softcover reprint of the hardcover 1st edition 1987 ISBN-13: 978-94-010-7916-7 e-ISBN-13: 978-94-009-3151-0 DO1: 10.1007/978-94-009-3151-0 All rights reserved. No part of this book may be reprinted, or reproduced or utilized in any form or by any electronic, mechanical or other means, now known or hereafter invented, including photocopying and recording, or in any information storage and retrieval system, without permission in writing from the publisher. British Library Cataloguing in Publication Data Mey凹, Sergei V. Fundamentals of palaeobotany. 1. Palaeobotany I. Title 11. Osnovy paleobotaniki. English 561 QE905 Library 01 Congress Catα loging in Publication Data Mey凹, Sergei Viktorovich. Fundamentals of palaeobotany. Bibliography: p. Includes index. 1. Paleobotany. I. Title. QE904.AIM45 561 8ι13000 Contents Foreword page xi Introduction xvii Acknowledgements xx Abbreviations xxi 1. Preservation 抄'pes αnd techniques of study of fossil plants 1 2. Principles of typology and of nomenclature of fossil plants 5 Parataxa and eutaxa S Taxa and characters 8 Peculiarity of the taxonomy and nomenclature of fossil plants 11 The binary (dual) system of fossil plants 12 The reasons for the inflation of generic na,mes 13 The species problem in palaeobotany lS The polytypic concept of the species 17 Assemblage-genera and assemblage-species 17 The cladistic methods 18 3. -
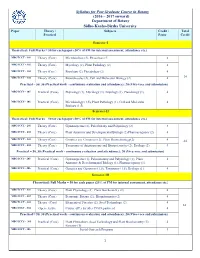
Syllabus for Post Graduate Course in Botany (2016 – 2017 Onward)
Syllabus for Post Graduate Course in Botany (2016 – 2017 onward) Department of Botany Sidho-Kanho-Birsha University Paper Theory / Subjects Credit / Total Practical Paper Credit Semester-I Theoretical: Full Marks = 50 for each paper (20% of FM for internal assessment, attendance etc.) MBOTCCT - 101 Theory (Core) Microbiology (2), Phycology (2) 4 MBOTCCT - 102 Theory (Core) Mycology (2), Plant Pathology (2) 4 MBOTCCT - 103 Theory (Core) Bryology (2), Pteridology (2) 4 MBOTCCT - 104 Theory (Core) Biomolecules (2), Cell and Molecular Biology (2) 4 24 Practical = 50, 30 (Practical work - continuous evaluation and attendance); 20 (Viva-voce and submission) MBOTCCS - 105 Practical (Core) Phycology (1), Mycology (1), Bryology (1), Pteridology (1). 4 MBOTCCS - 106 Practical (Core) Microbiology (1.5), Plant Pathology (1), Cell and Molecular 4 Biology (1.5). Semester-II Theoretical: Full Marks = 50 for each paper (20% of FM for internal assessment, attendance etc.) MBOTCCT - 201 Theory (Core) Gymnosperms (2), Paleobotany and Palynology (2) 4 MBOTCCT - 202 Theory (Core) Plant Anatomy and Developmental Biology (2) Pharmacognosy (2) 4 MBOTCCT - 203 Theory (Core) Genetics and Genomics (2), Plant Biotechnology(2) 4 24 MBOTCCT - 204 Theory (Core) Taxonomy of Angiosperms and Biosystematics (2), Ecology (2) 4 Practical = 50, 30 (Practical work - continuous evaluation and attendance); 20 (Viva-voce and submission) MBOTCCS - 205 Practical (Core) Gymnosperms (1), Palaeobotany and Palynology (1), Plant 4 Anatomy & Developmental Biology (1), Pharmacognosy (1). MBOTCCS - 206 Practical (Core) Genetics and Genomics (1.5), Taxonomy (1.5), Ecology (1). 4 Semester-III Theoretical: Full Marks = 50 for each paper (20% of FM for internal assessment, attendance etc.) MBOTCCT - 301 Theory (Core) Plant Physiology (2), Plant Biochemistry (2) 4 MBOTCCT - 302 Theory (Core) Economic Botany (2), Bioinformatics (2) 4 MBOTCCT - 303 Theory (Core) Elements of Forestry (2), Seed Technology (2). -

Evolução Das Plantas
EVOLUÇÃO DAS PLANTAS DAS EVOLUÇÃO Esta viagem pela Terra, pela sua formação, pelas suas primeiras atmosferas O segundo volume da coleção «Botânica em e vidas, pela evolução das plantas através das sucessivas mudanças é uma Português» faz uma síntese da história evolutiva leitura fascinante, às vezes difícil, mas como é mostrada e explicada com das plantas, desde a evolução da vida celular nas fontes grande sabedoria transmite conhecimento – saber científico –, que, apesar hidrotermais alcalinas oceânicas, há cerca de quatro EVOLUÇÃO das nossas falhas, conseguimos apreender e aprender. mil milhões de anos, até às grandes florestas tropicais hiperdiversas atuais. Como surgiu a fotossíntese? É uma lição de história, geologia, geografia, climatologia, agronomia As plantas nasceram na água: como invadiram a terra? e biologia, com notas de química e de física, cálculos matemáticos De que modo as plantas interagiram com a atmosfera DAS PLANTAS e paisagísticos, ou seja, a completa Aula de Botânica. terrestre? O que é e qual a origem do solo? Quais as funções das flores, esporos e sementes? Por que razão Carlos Aguiar Neste livro, desde a Pangeia, com a separação dos continentes, até hoje, as plantas com flor são tão bem-sucedidas? De que passando pelas várias erupções, avanços e recuos do mar, degelos e modo as megaextinções influenciaram a evolução das aquecimentos globais, vamos acompanhando os diferentes habitats, a | plantas? Estaremos perante uma nova megaextinção? Carlos Aguiar evolução e transformação das plantas pelos diversos continentes e mares Estas e muitas outras perguntas são respondidas – por seleção natural ou deriva genética – e como se foram aclimatando, ao longo deste livro. -

Fossil and Living Cycads Say No More Megasporophylls
hology orp a Miao et al., J Morphol Anat 2017, 1:2 nd M f A o n l a a t n o r m u y o J Journal of Morphology and Anatomy Research Article Article Open Access Fossil and Living Cycads Say "No More Megasporophylls" Yuyan Miao1,2, Zhong-Jian Liu3, Meina Wang3,4 and Xin Wang5* 1Beijing Museum of Natural History, Beijing, China 2State Key Laboratory of Biogeology and Environmental Geology, China University of Geosciences (Wuhan), Wuhan, China 3Shenzhen Key Laboratory for Orchid Conservation and Utilization, National Orchid Conservation Center of China and Orchid Conservation and Research Center of Shenzhen, Shenzhen, China 4College of Landscape Architecture, Fujian Agriculture and Forestry University, Fuzhou, China 5CAS Key Laboratory of Economic Stratigraphy and Paleogeography, Nanjing Institute of Geology and Palaeontology, Nanjing, China Abstract The origins of angiosperms and cycads are still mysterious. To understand the evolution of these groups as well as other gymnosperms it was impossible without mentioning a frequently used term “megasporophyll”. “Megasporophyll” is a concept that has been used widely in botany. This term is more or less related with the famous saying “Alles ist Blatt” by Goethe. This term became popular since Arber and Parkin hypothesized that the carpels in the Magnoliales were equivalent to and derived from former foliar parts bearing ovules along their margins (“megasporophyll”). Many botanists uncritically called the parts in all the reproductive organs of seed plants as “sporophylls”, no matter what they actually saw in the plants. However, the fact is that none of the reproductive parts (fossil or living), except those in the Cycadales, are foliar or leaf-like. -

Caytoniales Name of Teacher – Smt
Subject – Gymnosperms Topic – Caytoniales Name of teacher – Smt . Sibi O.S. Academic year – 2020 – 2021 ➢ Caytoniales were a small group of extinct gymnospermic plants. ➢ First described by Hamshaw Thomas in 1925 from late Triassic period. ➢ Caytonia is a berry like cupules with numerous small seeds. Examples of Caytoniales ➢ Leaves : Sagenopteris ➢ Microsporophyll : Caytonanthus ➢ Megasporophylls: Caytonia and Gristhorpia General characteristics ➢ Caytoniales were small branched trees or shrubs. ➢ Leaves ( Sagenopteris ). ➢ Leaves petiolate. ➢ Petiole slender with 3 to 6 terminal leaflets. ➢ Leaflets arrangement was palmate in pairs. Sagenopteris ➢ Each leaflet with distinct midrib. ➢ Leaf margin smooth with an acute apex. ➢ Venation similar to glossopteris. ➢ Upper and lower epidermis with thick cuticle. ➢ Stomatal development haplocheilic. ➢ Mesophyll differentiated into palisade and transfusion tissue. ➢ Leaflets fall by the formation of abscission layers , it is an Angiospermic character . ➢ Caytoniales had fertile branches with seed bearing cupules. ➢ Ovules were located inside the fleshy cupules with tough outer cuticle . ➢ Outer layers of the cupules were fleshy and fruit – like. ➢ Individual ovules had an apical tube like structure called micropylar canal. ➢ Mature ovule resembles a blueberry fruit. ➢ The extra protection of seeds in Caytoniales indicates they were predecessors of angiosperms Microsporophyll ➢ Example : Caytonanthus ➢ Microsporophyll consists of dorsi – ventral and pinnate rachis. ➢ Each rachis bears pinnate on either side. ➢ Each pinnae branches irregularly. ➢ The ultimate branches of pinnae bear the synangia. ➢ Each branch bears two sporangia terminally. ➢ Each sporangium was with four pollen sacs . ➢ Pollen grains were produced in the pollen sacs in groups of four. ➢ Pollen grains were small , shape similar to that of pine trees ➢ Pollen grains winged. ➢ Pollination is achieved through the wind. -

1 Relationships of Angiosperms To
Relationships of Angiosperms to 1 Other Seed Plants Seed plants are of fundamental importance both evolution- all gymnosperms (living and extinct) together are not arily and ecologically. They dominate terrestrial landscapes, monophyletic. Importantly, several fossil lineages, Cayto- and the seed has played a central role in agriculture and hu- niales, Bennettitales, Pentoxylales, and Glossopteridales man history. There are fi ve extant lineages of seed plants: (glossopterids), have been proposed as putative close rela- angiosperms, cycads, conifers, gnetophytes, and Ginkgo. tives of the angiosperms based on phylogenetic analyses These fi ve groups have usually been treated as distinct (e.g., Crane 1985; Rothwell and Serbet 1994; reviewed in phyla — Magnoliophyta (or Anthophyta), Cycadophyta, Doyle 2006, 2008, 2012; Friis et al. 2011). These fossil lin- Co ni fe ro phyta, Gnetophyta, and Ginkgophyta, respec- eages, sometimes referred to as the para-angiophytes, will tively. Cantino et al. (2007) used the following “rank- free” therefore be covered in more detail later in this chapter. An- names (see Chapter 12): Angiospermae, Cycadophyta, other fossil lineage, the corystosperms, has been proposed Coniferae, Gnetophyta, and Ginkgo. Of these, the angio- as a possible angiosperm ancestor as part of the “mostly sperms are by far the most diverse, with ~14,000 genera male hypothesis” (Frohlich and Parker 2000), but as re- and perhaps as many as 350,000 (The Plant List 2010) to viewed here, corystosperms usually do not appear as close 400,000 (Govaerts 2001) species. The conifers, with ap- angiosperm relatives in phylogenetic trees. proximately 70 genera and nearly 600 species, are the sec- The seed plants represent an ancient radiation, with ond largest group of living seed plants. -

Gymnosperms from the Middle Triassic of Antarctica: the First Structurally Preserved Cycad Pollen Cone
Int. J. Plant Sci. 164(6):1007–1020. 2003. ᭧ 2003 by The University of Chicago. All rights reserved. 1058-5893/2003/16406-0016$15.00 GYMNOSPERMS FROM THE MIDDLE TRIASSIC OF ANTARCTICA: THE FIRST STRUCTURALLY PRESERVED CYCAD POLLEN CONE Sharon D. Klavins,* Edith L. Taylor,* Michael Krings,† and Thomas N. Taylor* *Department of Ecology and Evolutionary Biology and Natural History Museum and Biodiversity Research Center, University of Kansas, Lawrence, Kansas 66045-7534, U.S.A.; and †Bayerische Staatssammlung fu¨r Pala¨ontologie und Geologie, Funktionseinheit Pala¨ontologie, Richard-Wagner-Strasse 10, 80333 Munich, Germany The first permineralized cycad pollen cone is described from the lower Middle Triassic of Antarctica. The cone is characterized by helically arranged, wedge-shaped microsporophylls, each with five or more spinelike projections extending from the rhomboid distal face. The vascular cylinder is dissected and produces paired traces to each microsporophyll. Three vascular bundles enter the base of the microsporophyll and divide to produce at least five vascular strands in the sporophyll lamina. Pollen sacs occur in two radial clusters near the lateral margins on the abaxial surface of the microsporophyll. Each cluster bears up to eight elongate pollen sacs that are fused for approximately half their length and display longitudinal dehiscence. Pollen sacs are sessile and attached to a vascularized, receptacle-like pad of tissue that is raised from the surface of the microsporophyll. Pollen is ovoid, psilate, and monosulcate. Although the affinities of this cone with the Cycadales are obvious, the complement of characters in the fossil is unique and thus does not permit assignment to an extant family. -

In Situ Gymnosperm Pollen from the Middle Jurassic of Yorkshire
In situ Gymnosperm pollen from the Middle Jurassic of Yorkshire Johanna+H.A. van Konijnenburg-van+Cittert Botanisch Museum en Herbarium, Utrecht. AfdelingPalaeobotanie en Pollenmorphologie SUMMARY In this paper the morphology of pollen grains yielded by male Gymnosperm fructifications from the Jurassic flora of Yorkshire is studied and discussed. Several new male fructifications were found and described: Hastystrobus gen. nov. was erected for male cones yielding the Eucommiidites type of pollen grains. This genus is mono- typic and the type species Hastystrobus muirii yielded pollen grains that agree with Eucommii- dites troedssonii. Hastystrobus muirii very probably has Cycadalean affinities, because the whole abaxial surface of the microsporophylls is covered with sporangia. For the first time the male fructification of Ginkgo huttoni (Heer) Sternberg is described. It resembles in general the male fructification of the recent Ginkgo biloba L., and the pollen with those of biloba. grains agree Ginkgo Male cones associated with Brachyphyllum crucis Kendall have been found and described. They yielded pollen grains that after short maceration were identifiable as Circulina, while after prolonged maceration they could be assigned to Classopollis multistriatus Burger. Brachyphyllum crucis is provisionally assigned to the Hirmerella- group on the basis of its male cone and pollen grains. The cones were compared with other male cones containing which also attributed the Hirmerella- It is that Classopollis pollen, were to group. suggested all members of the Hirmerella- have with of group an epidermis a special type stoma. Masculostrobus harrisii is described. This male resembles the male sp. nov. cone closely cone of Elatides williamsoni (Brgt) Sew., but its pollen grains are of the Inaperturopollenites-type, instead of the Perinopollenites-type. -

Taxonomical Revision of the Collection of Jurassic Plants from Roverè Di
Bollettino della Società Paleontologica Italiana, 48 (1), 2009, 1-13. Modena, 15 maggio 20091 Taxonomical revision of the Collection of Jurassic plants from Roverè di Velo (Veneto, northern Italy) stored in the Palaeontological Museum of the University of Naples “Federico II” Antonello BARTIROMO & Maria Rosaria BARONE LUMAGA A. Bartiromo, Dipartimento di Scienze della Terra, Università degli Studi di Napoli “Federico II”, Largo San Marcellino 10, I-80138 Napoli, Italy; [email protected] M.R. Barone Lumaga, Orto Botanico, Università degli Studi di Napoli “Federico II” Via Foria 223, I-80139, Napoli, Italy; [email protected] KEY WORDS - Palaeobotanical collection, Jurassic, Roverè di Velo, Palaeontological Museum of the University of Naples. ABSTRACT - This paper presents a revision of the Jurassic fossil plants collection from Roverè di Velo (Verona Province) housed in the Palaeontological Museum of the “Federico II” University of Naples. It was extremely difficult to review the entries of the Roverè di Velo plant fossils stored in the Museum. Indeed, only by finding ancient purchase inventories of the former Museum of Geology pre-dating the foundation of the Palaeontological Museum, we got enough information to pursue our purposes. Using these inventories, it is now possible to know the Museum of Geology purchased the Roverè di Velo fossil plants in 1874. Yet, only partial cataloguing of this collection was carried out, since. In the course of this study the provisional classification carried out in the second half of the XIX century was completely revised, and a number of additional specimens have been identified and catalogued, accordingly. Owing to the lack of cuticle, we had ascertained taxonomic affiliations relying on macroscopic features, only. -

Emese Réka Bodor
Növényi reproduktív képletek a Mecseki Kőszén Formációból DOKTORI ÉRTEKEZÉS 2015 BODOR EMESE RÉKA ELTE TTK, Őslénytani Tanszék MFGI, Földtani és Geofizikai Gyűjteményi Főosztály Témavezetők: Dr. BARBACKA MARIA (Főmuzeológus, MTM, Növénytár) Dr. KÁZMÉR MIKLÓS (Tanszékvezető egyetemi tanár, ELTE, TTK, Őslénytani Tanszék) ELTE, TTK, Földtudományi Doktori Iskola Doktori iskola vezetője: Dr. NEMES- NAGY JÓZSEF Földtan Geofizika Programvezetője: Dr. MINDSZENTY ANDREA Bodor Emese Réka Növényi reproduktív képletek a Mecseki Kőszén Formációból Tartalomjegyzék A KUTATÁSI TÉMA ELŐZMÉNYEI ÉS CÉLKITŰZÉSEI .......................................................................... 3 FÖLDTANI HÁTTÉR .......................................................................................................................................... 5 ANYAG ÉS MÓDSZEREK ................................................................................................................................. 9 VIZSGÁLT ANYAG ............................................................................................................................................... 9 ANYAGVIZSGÁLATI MÓDSZEREK ......................................................................................................................... 9 Kutikula vizsgálati eljárások ......................................................................................................................... 9 SZEDIMENTOLÓGIAI VIZSGÁLATI MÓDSZEREK .................................................................................................