A New Early Liassic Caytoniales Fructification from Hungary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Palaeoenvironmental Reconstruction of the Middle Jurassic of Sardinia (Italy) Based on Integrated Palaeobotanical, Palynological and Lithofacies Data Assessment
Palaeobio Palaeoenv DOI 10.1007/s12549-017-0306-z ORIGINAL PAPER A palaeoenvironmental reconstruction of the Middle Jurassic of Sardinia (Italy) based on integrated palaeobotanical, palynological and lithofacies data assessment Luca Giacomo Costamagna1 & Evelyn Kustatscher2,3 & Giovanni Giuseppe Scanu1 & Myriam Del Rio1 & Paola Pittau1 & Johanna H. A. van Konijnenburg-van Cittert4,5 Received: 15 May 2017 /Accepted: 19 September 2017 # The Author(s) 2017. This article is an open access publication Abstract During the Jurassic, Sardinia was close to con- diverse landscape with a variety of habitats. Collection- tinental Europe. Emerged lands started from a single is- and literature-based palaeobotanical, palynological and land forming in time a progressively sinking archipelago. lithofacies studies were carried out on the Genna Selole This complex palaeogeographic situation gave origin to a Formation for palaeoenvironmental interpretations. They evidence a generally warm and humid climate, affected occasionally by drier periods. Several distinct ecosystems can be discerned in this climate, including alluvial fans This article is a contribution to the special issue BJurassic biodiversity and with braided streams (Laconi-Gadoni lithofacies), paralic ^ terrestrial environments . swamps and coasts (Nurri-Escalaplano lithofacies), and lagoons and shallow marine environments (Ussassai- * Evelyn Kustatscher [email protected] Perdasdefogu lithofacies). The non-marine environments were covered by extensive lowland and a reduced coastal Luca Giacomo Costamagna and tidally influenced environment. Both the river and the [email protected] upland/hinterland environments are of limited impact for Giovanni Giuseppe Scanu the reconstruction. The difference between the composi- [email protected] tion of the palynological and palaeobotanical associations evidence the discrepancies obtained using only one of those Myriam Del Rio [email protected] proxies. -

Cretaceous Deposits and Flora of the Muravyov Amurskii Peninsula
ISSN 08695938, Stratigraphy and Geological Correlation, 2015, Vol. 23, No. 3, pp. 281–299. © Pleiades Publishing, Ltd., 2015. Original Russian Text © E.B. Volynets, 2015, published in Stratigrafiya. Geologicheskaya Korrelyatsiya, 2015, Vol. 23, No. 3, pp. 50–68. Cretaceous Deposits and Flora of the MuravyovAmurskii Peninsula (Amur Bay, Sea of Japan) E. B. Volynets Institute of Biology and Soil Science, Far East Branch, Russian Academy of Sciences, pr. 100letiya Vladivostoka 159, Vladivostok, 690022 Russia email: [email protected] Received August 21, 2013; in final form, March 24, 2014 Abstract—The Cretaceous sections and plant macrofossils are investigated in detail near Vladivostok on the MuravyovAmurskii Peninsula of southern Primorye. It is established that the Ussuri and Lipovtsy forma tions in the reference section of the Markovskii Peninsula rest with unconformity upon Upper Triassic strata. The continuous Cretaceous succession is revealed in the Peschanka River area of the northern Muravyov Amurskii Peninsula, where plant remains were first sampled from the lower and upper parts of the Korkino Group, which are determined to be the late Albian–late Cenmanian in age. The taxonomic composition of floral assemblages from the Ussuri, Lipovtsy, and Galenki formations is widened owing to additional finds of plant remains. The Korkino Group received floral characteristics for the first time. The Cretaceous flora of the peninsula is represented by 126 taxa. It is established that ferns and conifers are dominant elements of the Ussuri floral assemblage, while the Lipovtsy Assemblage is dominated by ferns, conifers, and cycadphytes. In addition, the latter assemblage is characterized by the highest taxonomic diversity. The Galenki Assemblage is marked by the first appearance of rare flowering plants against the background of dominant ferns and coni fers. -

The Lower Cretaceous Flora of the Gates Formation from Western Canada
The Lower Cretaceous Flora of the Gates Formation from Western Canada A Shesis Submitted to the College of Graduate Studies and Research in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy in the Department of Geological Sciences Univ. of Saska., Saskatoon?SI(, Canada S7N 3E2 b~ Zhihui Wan @ Copyright Zhihui Mian, 1996. Al1 rights reserved. National Library Bibliothèque nationale 1*1 of Canada du Canada Acquisitions and Acquisitions et Bibliographic Services services bibliographiques 395 Wellington Street 395. rue Wellington Ottawa ON KlA ON4 Ottawa ON K1A ON4 Canada Canada The author has granted a non- L'auteur a accordé une licence non exclusive licence allowing the exclusive permettant à la National Libraxy of Canada to Bibliothèque nationale du Canada de reproduce, loan, distribute or sell reproduire, prêter, distribuer ou copies of this thesis in microfom, vendre des copies de cette thèse sous paper or electronic formats. la fome de microfiche/nlm, de reproduction sur papier ou sur foxmat électronique. The author retains ownership of the L'auteur conserve la propriété du copyright in this thesis. Neither the droit d'auteur qui protège cette thèse. thesis nor substantial extracts fiom it Ni la thèse ni des extraits substantiels may be printed or otherwise de celle-ci ne doivent être imprimés reproduced without the author's ou autrement reproduits sans son permission. autorisation. College of Graduate Studies and Research SUMMARY OF DISSERTATION Submitted in partial fulfillment of the requirernents for the DEGREE OF DOCTOR OF PHILOSOPHY ZHIRUI WAN Depart ment of Geological Sciences University of Saskatchewan Examining Commit tee: Dr. -

Palynology of the Upper Chinle Formation in Northern New Mexico, U.S.A
Lindström et al. 1 1 Palynology of the upper Chinle Formation in northern New Mexico, U.S.A.: 2 implications for biostratigraphy and terrestrial ecosystem change during the Late 3 Triassic (Norian–Rhaetian) 4 a* b c d 5 Sofie Lindström , Randall B. Irmis , Jessica H. Whiteside , Nathan D. Smith , Sterling J. e f 6 Nesbitt , and Alan H. Turner 7 a 8 Geological Survey of Denmark and Greenland, Øster Voldgade 10, DK-1350 Copenhagen 9 K, DENMARK, [email protected] b 10 Natural History Museum of Utah and Department of Geology & Geophysics, University of 11 Utah, Salt Lake City, UT 84108-1214, USA c 12 Ocean and Earth Science, National Oceanography Centre Southampton, University of 13 Southampton, European Way, Southampton SO14 3ZH, UNITED KINGDOM d 14 Dinosaur Institute, Natural History Museum of Los Angeles County, Los Angeles, CA 15 90007, USA e 16 Department of Geosciences, Virginia Polytechnic Institute and State University, Blacksburg, 17 Virginia 24601 USA f 18 Department of Anatomical Sciences, Stony Brook University, Stony Brook, New York 19 11794-8081, USA 20 21 Abstract 22 A new densely sampled palynological record from the vertebrate-bearing upper Chinle 23 Formation at Ghost Ranch in the Chama Basin of northwestern New Mexico provides insights 24 into the biostratigraphy and terrestrial ecosystem changes during the Late Triassic of 25 northwestern Pangaea. Spore-pollen assemblages from the Poleo Sandstone, Petrified Forest, Lindström et al. 2 26 and 'siltstone' members are dominated by pollen of corystospermous seed ferns (Alisporites) 27 and voltziacean conifers (Enzonalasporites, Patinasporites). Other abundant taxa include 28 Klausipollenites gouldii and the enigmatic fused tetrad Froelichsporites traversei, whereas 29 spores of ferns and fern allies are generally rare. -

JUDD W.S. Et. Al. (2002) Plant Systematics: a Phylogenetic Approach. Chapter 7. an Overview of Green
UNCORRECTED PAGE PROOFS An Overview of Green Plant Phylogeny he word plant is commonly used to refer to any auto- trophic eukaryotic organism capable of converting light energy into chemical energy via the process of photosynthe- sis. More specifically, these organisms produce carbohydrates from carbon dioxide and water in the presence of chlorophyll inside of organelles called chloroplasts. Sometimes the term plant is extended to include autotrophic prokaryotic forms, especially the (eu)bacterial lineage known as the cyanobacteria (or blue- green algae). Many traditional botany textbooks even include the fungi, which differ dramatically in being heterotrophic eukaryotic organisms that enzymatically break down living or dead organic material and then absorb the simpler products. Fungi appear to be more closely related to animals, another lineage of heterotrophs characterized by eating other organisms and digesting them inter- nally. In this chapter we first briefly discuss the origin and evolution of several separately evolved plant lineages, both to acquaint you with these important branches of the tree of life and to help put the green plant lineage in broad phylogenetic perspective. We then focus attention on the evolution of green plants, emphasizing sev- eral critical transitions. Specifically, we concentrate on the origins of land plants (embryophytes), of vascular plants (tracheophytes), of 1 UNCORRECTED PAGE PROOFS 2 CHAPTER SEVEN seed plants (spermatophytes), and of flowering plants dons.” In some cases it is possible to abandon such (angiosperms). names entirely, but in others it is tempting to retain Although knowledge of fossil plants is critical to a them, either as common names for certain forms of orga- deep understanding of each of these shifts and some key nization (e.g., the “bryophytic” life cycle), or to refer to a fossils are mentioned, much of our discussion focuses on clade (e.g., applying “gymnosperms” to a hypothesized extant groups. -

A Stable Isotopic Investigation of Resource Partitioning Among Neosauropod Dinosaurs of the Upper Jurassic Morrison Formation
A stable isotopic investigation of resource partitioning among neosauropod dinosaurs of the Upper Jurassic Morrison Formation Benjamin T. Breeden, III SID: 110305422 [email protected] GEOL394H University of Maryland, College Park, Department of Geology 29 April 2011 Advisors: Dr. Thomas R. Holtz1, Jr., Dr. Alan Jay Kaufman1, and Dr. Matthew T. Carrano2 1: University of Maryland, College Park, Department of Geology 2: National Museum of Natural History, Department of Paleobiology ABSTRACT For more than a century, morphological studies have been used to attempt to understand the partitioning of resources in the Morrison Fauna, particularly between members of the two major clades of neosauropod (long-necked, megaherbivorous) dinosaurs: Diplodocidae and Macronaria. While it is generally accepted that most macronarians fed 3-5m above the ground, the feeding habits of diplodocids are somewhat more enigmatic; it is not clear whether diplodocids fed higher or lower than macronarians. While many studies exploring sauropod resource portioning have focused on differences in the morphologies of the two groups, few have utilized geochemical evidence. Stable isotope geochemistry has become an increasingly common and reliable means of investigating paleoecological questions, and due to the resistance of tooth enamel to diagenetic alteration, fossil teeth can provide invaluable paleoecological and behavioral data that would be otherwise unobtainable. Studies in the Ituri Rainforest in the Democratic Republic of the Congo, have shown that stable isotope ratios measured in the teeth of herbivores reflect the heights at which these animals fed in the forest due to isotopic variation in plants with height caused by differences in humidity at the forest floor and the top of the forest exposed to the atmosphere. -

Dr. Sahanaj Jamil Associate Professor of Botany M.L.S.M. College, Darbhanga
Subject BOTANY Paper No V Paper Code BOT521 Topic Taxonomy and Diversity of Seed Plant: Gymnosperms & Angiosperms Dr. Sahanaj Jamil Associate Professor of Botany M.L.S.M. College, Darbhanga BOTANY PG SEMESTER – II, PAPER –V BOT521: Taxonomy and Diversity of seed plants UNIT- I BOTANY PG SEMESTER – II, PAPER –V BOT521: Taxonomy and Diversity of seed plants Classification of Gymnosperms. # Robert Brown (1827) for the first time recognized Gymnosperm as a group distinct from angiosperm due to the presence of naked ovules. BENTHAM and HOOKSER (1862-1883) consider them equivalent to dicotyledons and monocotyledons and placed between these two groups of angiosperm. They recognized three classes of gymnosperm, Cyacadaceae, coniferac and gnetaceae. Later ENGLER (1889) created a group Gnikgoales to accommodate the genus giankgo. Van Tieghem (1898) treated Gymnosperm as one of the two subdivision of spermatophyte. To accommodate the fossil members three more classes- Pteridospermae, Cordaitales, and Bennettitales where created. Coulter and chamberlain (1919), Engler and Prantl (1926), Rendle (1926) and other considered Gymnosperm as a division of spermatophyta, Phanerogamia or Embryoptyta and they further divided them into seven orders: - i) Cycadofilicales ii) Cycadales iii) Bennettitales iv) Ginkgoales v) Coniferales vi) Corditales vii) Gnetales On the basis of wood structure steward (1919) divided Gymnosperm into two classes: - i) Manoxylic ii) Pycnoxylic The various classification of Gymnosperm proposed by various workers are as follows: - i) Sahni (1920): - He recognized two sub-divison in gymnosperm: - a) Phylospermae b) Stachyospermae BOTANY PG SEMESTER – II, PAPER –V BOT521: Taxonomy and Diversity of seed plants ii) Classification proposed by chamber lain (1934): - He divided Gymnosperm into two divisions: - a) Cycadophyta b) Coniterophyta iii) Classification proposed by Tippo (1942):- He considered Gymnosperm as a class of the sub- phylum pteropsida and divided them into two sub classes:- a) Cycadophyta b) Coniferophyta iv) D. -

Biodiversity and the Reconstruction of Early Jurassic Flora from the Mecsek
Acta Palaeobotanica 51(2): 127–179, 2011 Biodiversity and the reconstruction of Early Jurassic fl ora from the Mecsek Mountains (southern Hungary) MARIA BARBACKA Hungarian Natural History Museum, Department of Botany, H-1476, P.O. Box 222, W. Szafer Institute of Botany, Polish Academy of Sciences, Lubicz 46, 31-512 Kraków, Poland; e-mail: [email protected] Received 15 June 2011; accepted for publication 27 October 2011 ABSTRACT. Rich material from Hungary’s Early Jurassic (the Mecsek Mts.) was investigated in a palaeoen- vironmental context. The locality (or, more precisely, area with a number of fossiliferous sites) is known as a delta plain, showing diverse facies, which suggest different landscapes with corresponding plant assemblages. Taphonomical observations proved that autochthonous and parautochthonous plant associations were present. The reconstruction of the biomes is based on the co-occurrence of taxa and their connection with the rock matrix and sites in the locality, as well as the environmental adaptation of the plants expressed in their morphology and cuticular structure. The climatic parameters were confi rmed as typical for the Early Jurassic by resolution of a palaeoatmospheric CO2 level based on the stomatal index of one of the common species, Ginkgoites mar- ginatus (Nathorst) Florin. Plant communities were differentiated with the help of Detrended Correspondence Analysis (DCA); the rela- tionship between taxa and sites and lithofacies and sites, were analysed by Ward’s minimal variance and cluste- red with the help -

Ecological Sorting of Vascular Plant Classes During the Paleozoic Evolutionary Radiation
i1 Ecological Sorting of Vascular Plant Classes During the Paleozoic Evolutionary Radiation William A. DiMichele, William E. Stein, and Richard M. Bateman DiMichele, W.A., Stein, W.E., and Bateman, R.M. 2001. Ecological sorting of vascular plant classes during the Paleozoic evolutionary radiation. In: W.D. Allmon and D.J. Bottjer, eds. Evolutionary Paleoecology: The Ecological Context of Macroevolutionary Change. Columbia University Press, New York. pp. 285-335 THE DISTINCTIVE BODY PLANS of vascular plants (lycopsids, ferns, sphenopsids, seed plants), corresponding roughly to traditional Linnean classes, originated in a radiation that began in the late Middle Devonian and ended in the Early Carboniferous. This relatively brief radiation followed a long period in the Silurian and Early Devonian during wrhich morphological complexity accrued slowly and preceded evolutionary diversifications con- fined within major body-plan themes during the Carboniferous. During the Middle Devonian-Early Carboniferous morphological radiation, the major class-level clades also became differentiated ecologically: Lycopsids were cen- tered in wetlands, seed plants in terra firma environments, sphenopsids in aggradational habitats, and ferns in disturbed environments. The strong con- gruence of phylogenetic pattern, morphological differentiation, and clade- level ecological distributions characterizes plant ecological and evolutionary dynamics throughout much of the late Paleozoic. In this study, we explore the phylogenetic relationships and realized ecomorphospace of reconstructed whole plants (or composite whole plants), representing each of the major body-plan clades, and examine the degree of overlap of these patterns with each other and with patterns of environmental distribution. We conclude that 285 286 EVOLUTIONARY PALEOECOLOGY ecological incumbency was a major factor circumscribing and channeling the course of early diversification events: events that profoundly affected the structure and composition of modern plant communities. -

Fundamentals of Palaeobotany Fundamentals of Palaeobotany
Fundamentals of Palaeobotany Fundamentals of Palaeobotany cuGU .叮 v FimditLU'φL-EjAA ρummmm 吋 eαymGfr 伊拉ddd仇側向iep M d、 況 O C O W Illustrations by the author uc削 ∞叩N Nn凹創 刊,叫MH h 咀 可 白 a aEE-- EEA First published in 1987 by Chapman αndHallLtd 11 New Fetter Lane, London EC4P 4EE Published in the USA by Chα~pman and H all 29 West 35th Street: New Yo地 NY 10001 。 1987 S. V. M秒len Softcover reprint of the hardcover 1st edition 1987 ISBN-13: 978-94-010-7916-7 e-ISBN-13: 978-94-009-3151-0 DO1: 10.1007/978-94-009-3151-0 All rights reserved. No part of this book may be reprinted, or reproduced or utilized in any form or by any electronic, mechanical or other means, now known or hereafter invented, including photocopying and recording, or in any information storage and retrieval system, without permission in writing from the publisher. British Library Cataloguing in Publication Data Mey凹, Sergei V. Fundamentals of palaeobotany. 1. Palaeobotany I. Title 11. Osnovy paleobotaniki. English 561 QE905 Library 01 Congress Catα loging in Publication Data Mey凹, Sergei Viktorovich. Fundamentals of palaeobotany. Bibliography: p. Includes index. 1. Paleobotany. I. Title. QE904.AIM45 561 8ι13000 Contents Foreword page xi Introduction xvii Acknowledgements xx Abbreviations xxi 1. Preservation 抄'pes αnd techniques of study of fossil plants 1 2. Principles of typology and of nomenclature of fossil plants 5 Parataxa and eutaxa S Taxa and characters 8 Peculiarity of the taxonomy and nomenclature of fossil plants 11 The binary (dual) system of fossil plants 12 The reasons for the inflation of generic na,mes 13 The species problem in palaeobotany lS The polytypic concept of the species 17 Assemblage-genera and assemblage-species 17 The cladistic methods 18 3. -
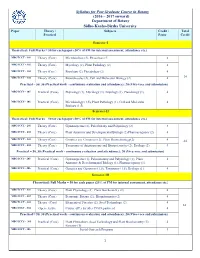
Syllabus for Post Graduate Course in Botany (2016 – 2017 Onward)
Syllabus for Post Graduate Course in Botany (2016 – 2017 onward) Department of Botany Sidho-Kanho-Birsha University Paper Theory / Subjects Credit / Total Practical Paper Credit Semester-I Theoretical: Full Marks = 50 for each paper (20% of FM for internal assessment, attendance etc.) MBOTCCT - 101 Theory (Core) Microbiology (2), Phycology (2) 4 MBOTCCT - 102 Theory (Core) Mycology (2), Plant Pathology (2) 4 MBOTCCT - 103 Theory (Core) Bryology (2), Pteridology (2) 4 MBOTCCT - 104 Theory (Core) Biomolecules (2), Cell and Molecular Biology (2) 4 24 Practical = 50, 30 (Practical work - continuous evaluation and attendance); 20 (Viva-voce and submission) MBOTCCS - 105 Practical (Core) Phycology (1), Mycology (1), Bryology (1), Pteridology (1). 4 MBOTCCS - 106 Practical (Core) Microbiology (1.5), Plant Pathology (1), Cell and Molecular 4 Biology (1.5). Semester-II Theoretical: Full Marks = 50 for each paper (20% of FM for internal assessment, attendance etc.) MBOTCCT - 201 Theory (Core) Gymnosperms (2), Paleobotany and Palynology (2) 4 MBOTCCT - 202 Theory (Core) Plant Anatomy and Developmental Biology (2) Pharmacognosy (2) 4 MBOTCCT - 203 Theory (Core) Genetics and Genomics (2), Plant Biotechnology(2) 4 24 MBOTCCT - 204 Theory (Core) Taxonomy of Angiosperms and Biosystematics (2), Ecology (2) 4 Practical = 50, 30 (Practical work - continuous evaluation and attendance); 20 (Viva-voce and submission) MBOTCCS - 205 Practical (Core) Gymnosperms (1), Palaeobotany and Palynology (1), Plant 4 Anatomy & Developmental Biology (1), Pharmacognosy (1). MBOTCCS - 206 Practical (Core) Genetics and Genomics (1.5), Taxonomy (1.5), Ecology (1). 4 Semester-III Theoretical: Full Marks = 50 for each paper (20% of FM for internal assessment, attendance etc.) MBOTCCT - 301 Theory (Core) Plant Physiology (2), Plant Biochemistry (2) 4 MBOTCCT - 302 Theory (Core) Economic Botany (2), Bioinformatics (2) 4 MBOTCCT - 303 Theory (Core) Elements of Forestry (2), Seed Technology (2). -

Evolução Das Plantas
EVOLUÇÃO DAS PLANTAS DAS EVOLUÇÃO Esta viagem pela Terra, pela sua formação, pelas suas primeiras atmosferas O segundo volume da coleção «Botânica em e vidas, pela evolução das plantas através das sucessivas mudanças é uma Português» faz uma síntese da história evolutiva leitura fascinante, às vezes difícil, mas como é mostrada e explicada com das plantas, desde a evolução da vida celular nas fontes grande sabedoria transmite conhecimento – saber científico –, que, apesar hidrotermais alcalinas oceânicas, há cerca de quatro EVOLUÇÃO das nossas falhas, conseguimos apreender e aprender. mil milhões de anos, até às grandes florestas tropicais hiperdiversas atuais. Como surgiu a fotossíntese? É uma lição de história, geologia, geografia, climatologia, agronomia As plantas nasceram na água: como invadiram a terra? e biologia, com notas de química e de física, cálculos matemáticos De que modo as plantas interagiram com a atmosfera DAS PLANTAS e paisagísticos, ou seja, a completa Aula de Botânica. terrestre? O que é e qual a origem do solo? Quais as funções das flores, esporos e sementes? Por que razão Carlos Aguiar Neste livro, desde a Pangeia, com a separação dos continentes, até hoje, as plantas com flor são tão bem-sucedidas? De que passando pelas várias erupções, avanços e recuos do mar, degelos e modo as megaextinções influenciaram a evolução das aquecimentos globais, vamos acompanhando os diferentes habitats, a | plantas? Estaremos perante uma nova megaextinção? Carlos Aguiar evolução e transformação das plantas pelos diversos continentes e mares Estas e muitas outras perguntas são respondidas – por seleção natural ou deriva genética – e como se foram aclimatando, ao longo deste livro.