An Advanced Peltasperm Permoxylocarpus Trojanus Naug
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Triassic) in Barreal Depocenter, San Juan Province, Argentina
Andean Geology ISSN: 0718-7092 ISSN: 0718-7106 [email protected] Servicio Nacional de Geología y Minería Chile Stratigraphical, sedimentological and palaeofloristic characterization of the Sorocayense Group (Triassic) in Barreal depocenter, San Juan Province, Argentina Bodnar, Josefina; Iglesias, Ari; Colombi, Carina E.; Drovandi, Juan Martín Stratigraphical, sedimentological and palaeofloristic characterization of the Sorocayense Group (Triassic) in Barreal depocenter, San Juan Province, Argentina Andean Geology, vol. 46, no. 3, 2019 Servicio Nacional de Geología y Minería, Chile Available in: https://www.redalyc.org/articulo.oa?id=173961656006 This work is licensed under Creative Commons Attribution 3.0 International. PDF generated from XML JATS4R by Redalyc Project academic non-profit, developed under the open access initiative Josefina Bodnar, et al. Stratigraphical, sedimentological and palaeofloristic characterization of ... Research article Stratigraphical, sedimentological and palaeofloristic characterization of the Sorocayense Group (Triassic) in Barreal depocenter, San Juan Province, Argentina Caracterización estratigráfica, sedimentológica y paleoflorística del Grupo Sorocayense (Triásico) en el área de Barreal, provincia de San Juan, Argentina Josefina Bodnar *12 Redalyc: https://www.redalyc.org/articulo.oa? Universidad Nacional de La Plata, Argentina id=173961656006 [email protected] Ari Iglesias 23 Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina [email protected] Carina E. Colombi 24 Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina [email protected] Juan Martín Drovandi 24 Consejo Nacional de Investigaciones Científicas y Técnicas, Argentina [email protected] Received: 30 November 2017 Accepted: 30 October 2018 Published: 04 February 2019 Abstract: e northern area of Cuyo Basin (west-central Argentina) corresponds to the Rincón Blanco half-graben, whose filling is arranged into the Rincón Blanco and Sorocayense groups. -

Reappraisal of the Genus Dicroidium Gothan from the Triassic Sediments of India
The Palaeobotanist 63(2014): 137–155 0031–0174/2014 Reappraisal of the genus Dicroidium Gothan from the Triassic sediments of India PANKAJ K. PAL1*, AMIT K. GHOSH2, RATAN KAR2, R.S. SINGH2, MANOBIKA SARKAR1 AND RESHMI CHATTERJEE2 1Department of Botany, UGC Centre of Advanced Study, University of Burdwan, Burdwan–713 104, West Bengal, India. 2Birbal Sahni Institute of Palaeobotany, 53 University Road, Lucknow 226 007, India. *Corresponding author: [email protected] (Received 28 August, 2014; revised version accepted 25 September, 2014) ABSTRACT Pal PK, Ghosh AK, Kar R, Singh RS, Sarkar M & Chatterjee R 2014. Reappraisal of the genus Dicroidium Gothan from the Triassic sediments of India. The Palaeobotanist 63(2): 137–155. The genus Dicroidium Gothan, belonging to Corystospermaceae, is characterised by pinnately compound leaves with proximally forked primary rachis. The genus was earlier included under the genus Thinnfeldia Ettingshausen. Dicroidium is the most consistent macrofloral element in the Triassic strata of Southern Hemisphere. The present reassessment deals with the morphotaxonomy and stratigraphic significance of the species of Dicroidium in India. A critical review of the literature reveals that the specimens of Dicroidium described so far from India require reassessment, because same morphotypes have often been placed under different species names and sometimes dissimilar elements have been assigned to the same species. In view of this, a thorough analysis of Indian Dicroidium was undertaken based on fresh collections along with the species described earlier by previous workers. The present reappraisal reveals that the genus in the Triassic of Peninsular India is represented by eight species. These are D. hughesii (Feistmantel) Lele, D. -

Andrew Leslie
Andrew Leslie [email protected] • (401) 863-5931 • andrewleslielab.com Department of Ecology and Evolutionary Biology • Brown University Box G-W, 80 Waterman Street • Providence, RI 02912 EDUCATION Ph.D., University of Chicago (Chicago, IL) 2010 Department of the Geophysical Sciences Dissertation: Forms following functions: exploring the evolution of morphological diversity in seed plant reproductive structures. C. Kevin Boyce (advisor), Peter Crane, David Jablonski, Michael LaBarbera, Manfred Rudat B.A. (with honors), University of Pennsylvania 2004 Geology (honors); Biochemistry (honors) Honors thesis: Leaf development in Carboniferous seed plants. Hermann Pfefferkorn (advisor) CURRENT APPOINTMENT Assistant Professor 2014-present Department of Ecology and Evolutionary Biology, Brown University RESEARCH EXPERIENCE Postdoctoral research associate, Yale University 2010-2014 Projects: Fossil conifer descriptions, conifer phylogenetics, conifer reproductive biology, molecular dating techniques, character evolution (advisors: Peter Crane, Michael Donoghue) Doctoral dissertation research, University of Chicago 2004-2010 Topics: Conifer evolution, pollination biology, functional morphology (advisor: C. Kevin Boyce) Research assistant, University of Chicago 2007-2009 Project: Cretaceous plant fossil descriptions from Upatoi Creek, Georgia (advisor: Peter Crane) Research assistant, University of North Carolina, Chapel Hill 2006 Project: Reproductive morphology of the Devonian plant Rhacophyton (advisor: Patricia Gensel) Leslie CV 1 Research -

JUDD W.S. Et. Al. (2002) Plant Systematics: a Phylogenetic Approach. Chapter 7. an Overview of Green
UNCORRECTED PAGE PROOFS An Overview of Green Plant Phylogeny he word plant is commonly used to refer to any auto- trophic eukaryotic organism capable of converting light energy into chemical energy via the process of photosynthe- sis. More specifically, these organisms produce carbohydrates from carbon dioxide and water in the presence of chlorophyll inside of organelles called chloroplasts. Sometimes the term plant is extended to include autotrophic prokaryotic forms, especially the (eu)bacterial lineage known as the cyanobacteria (or blue- green algae). Many traditional botany textbooks even include the fungi, which differ dramatically in being heterotrophic eukaryotic organisms that enzymatically break down living or dead organic material and then absorb the simpler products. Fungi appear to be more closely related to animals, another lineage of heterotrophs characterized by eating other organisms and digesting them inter- nally. In this chapter we first briefly discuss the origin and evolution of several separately evolved plant lineages, both to acquaint you with these important branches of the tree of life and to help put the green plant lineage in broad phylogenetic perspective. We then focus attention on the evolution of green plants, emphasizing sev- eral critical transitions. Specifically, we concentrate on the origins of land plants (embryophytes), of vascular plants (tracheophytes), of 1 UNCORRECTED PAGE PROOFS 2 CHAPTER SEVEN seed plants (spermatophytes), and of flowering plants dons.” In some cases it is possible to abandon such (angiosperms). names entirely, but in others it is tempting to retain Although knowledge of fossil plants is critical to a them, either as common names for certain forms of orga- deep understanding of each of these shifts and some key nization (e.g., the “bryophytic” life cycle), or to refer to a fossils are mentioned, much of our discussion focuses on clade (e.g., applying “gymnosperms” to a hypothesized extant groups. -

Exceptionally Well-Preserved Early Cretaceous Leaves of Nilssoniopteris from Central Mongolia
Acta Palaeobotanica 58(2): 135–157, 2018 e-ISSN 2082-0259 DOI: 10.2478/acpa-2018-0016 ISSN 0001-6594 Exceptionally well-preserved Early Cretaceous leaves of Nilssoniopteris from central Mongolia FABIANY HERRERA1,5,*, GONGLE SHI2, GOMBOSUREN TSOLMON3, NIIDEN ICHINNOROV 3, MASAMICHI TAKAHASHI4, PETER R. CRANE5,6 and PATRICK S. HERENDEEN1 1 Chicago Botanic Garden, Glencoe, Illinois 60022 USA; e-mails: [email protected]; [email protected] 2 State Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology and Center for Excellence in Life and Paleoenvironment, Chinese Academy of Sciences, Nanjing 210008 People’s Republic of China; e-mail: [email protected] 3 Institute of Paleontology and Geology, Mongolian Academy of Sciences, Ulaanbaatar-51, Mongolia; e-mails: [email protected]; [email protected] 4 Department of Environmental Sciences, Niigata University, Ikarashi, Nishi-ku, Niigata 950-2181 Japan; e-mail: [email protected] 5 Oak Spring Garden Foundation, Upperville, Virginia 20184 USA 6 School of Forestry and Environmental Studies, Yale University, New Haven, Connecticut 06511 USA; e-mail: [email protected] Received 20 June 2018; accepted for publication 25 October 2018 ABSTRACT. Two new Early Cretaceous (Aptian-Albian) species of fossil bennettitalean leaves are described from central Mongolia and assigned to the genus Nilssoniopteris. Nilssoniopteris tomentosa F.Herrera, G.Shi, Tsolmon, Ichinnorov, Takahashi, P.R.Crane, et Herend. sp. nov., isolated from bulk sediment samples collected for mesofossils in the Tevshiingovi Formation at the Tevshiin Govi opencast coal mine, is distinctive in having a dense, well-developed indumentum composed of branched, fattened multicellular trichomes on the abaxial leaf surface. -

Ecological Sorting of Vascular Plant Classes During the Paleozoic Evolutionary Radiation
i1 Ecological Sorting of Vascular Plant Classes During the Paleozoic Evolutionary Radiation William A. DiMichele, William E. Stein, and Richard M. Bateman DiMichele, W.A., Stein, W.E., and Bateman, R.M. 2001. Ecological sorting of vascular plant classes during the Paleozoic evolutionary radiation. In: W.D. Allmon and D.J. Bottjer, eds. Evolutionary Paleoecology: The Ecological Context of Macroevolutionary Change. Columbia University Press, New York. pp. 285-335 THE DISTINCTIVE BODY PLANS of vascular plants (lycopsids, ferns, sphenopsids, seed plants), corresponding roughly to traditional Linnean classes, originated in a radiation that began in the late Middle Devonian and ended in the Early Carboniferous. This relatively brief radiation followed a long period in the Silurian and Early Devonian during wrhich morphological complexity accrued slowly and preceded evolutionary diversifications con- fined within major body-plan themes during the Carboniferous. During the Middle Devonian-Early Carboniferous morphological radiation, the major class-level clades also became differentiated ecologically: Lycopsids were cen- tered in wetlands, seed plants in terra firma environments, sphenopsids in aggradational habitats, and ferns in disturbed environments. The strong con- gruence of phylogenetic pattern, morphological differentiation, and clade- level ecological distributions characterizes plant ecological and evolutionary dynamics throughout much of the late Paleozoic. In this study, we explore the phylogenetic relationships and realized ecomorphospace of reconstructed whole plants (or composite whole plants), representing each of the major body-plan clades, and examine the degree of overlap of these patterns with each other and with patterns of environmental distribution. We conclude that 285 286 EVOLUTIONARY PALEOECOLOGY ecological incumbency was a major factor circumscribing and channeling the course of early diversification events: events that profoundly affected the structure and composition of modern plant communities. -

Fundamentals of Palaeobotany Fundamentals of Palaeobotany
Fundamentals of Palaeobotany Fundamentals of Palaeobotany cuGU .叮 v FimditLU'φL-EjAA ρummmm 吋 eαymGfr 伊拉ddd仇側向iep M d、 況 O C O W Illustrations by the author uc削 ∞叩N Nn凹創 刊,叫MH h 咀 可 白 a aEE-- EEA First published in 1987 by Chapman αndHallLtd 11 New Fetter Lane, London EC4P 4EE Published in the USA by Chα~pman and H all 29 West 35th Street: New Yo地 NY 10001 。 1987 S. V. M秒len Softcover reprint of the hardcover 1st edition 1987 ISBN-13: 978-94-010-7916-7 e-ISBN-13: 978-94-009-3151-0 DO1: 10.1007/978-94-009-3151-0 All rights reserved. No part of this book may be reprinted, or reproduced or utilized in any form or by any electronic, mechanical or other means, now known or hereafter invented, including photocopying and recording, or in any information storage and retrieval system, without permission in writing from the publisher. British Library Cataloguing in Publication Data Mey凹, Sergei V. Fundamentals of palaeobotany. 1. Palaeobotany I. Title 11. Osnovy paleobotaniki. English 561 QE905 Library 01 Congress Catα loging in Publication Data Mey凹, Sergei Viktorovich. Fundamentals of palaeobotany. Bibliography: p. Includes index. 1. Paleobotany. I. Title. QE904.AIM45 561 8ι13000 Contents Foreword page xi Introduction xvii Acknowledgements xx Abbreviations xxi 1. Preservation 抄'pes αnd techniques of study of fossil plants 1 2. Principles of typology and of nomenclature of fossil plants 5 Parataxa and eutaxa S Taxa and characters 8 Peculiarity of the taxonomy and nomenclature of fossil plants 11 The binary (dual) system of fossil plants 12 The reasons for the inflation of generic na,mes 13 The species problem in palaeobotany lS The polytypic concept of the species 17 Assemblage-genera and assemblage-species 17 The cladistic methods 18 3. -

Diversity and Homologies of Corystosperm Seed-Bearing Structures from the Early Cretaceous of Mongolia Aã B,C D E F Gongle Shi , Peter R
Journal of Systematic Palaeontology ISSN: 1477-2019 (Print) 1478-0941 (Online) Journal homepage: https://www.tandfonline.com/loi/tjsp20 Diversity and homologies of corystosperm seed- bearing structures from the Early Cretaceous of Mongolia Gongle Shi, Peter R. Crane, Patrick S. Herendeen, Niiden Ichinnorov, Masamichi Takahashi & Fabiany Herrera To cite this article: Gongle Shi, Peter R. Crane, Patrick S. Herendeen, Niiden Ichinnorov, Masamichi Takahashi & Fabiany Herrera (2019) Diversity and homologies of corystosperm seed- bearing structures from the Early Cretaceous of Mongolia, Journal of Systematic Palaeontology, 17:12, 997-1029, DOI: 10.1080/14772019.2018.1493547 To link to this article: https://doi.org/10.1080/14772019.2018.1493547 Published online: 14 Jan 2019. Submit your article to this journal Article views: 142 View Crossmark data Full Terms & Conditions of access and use can be found at https://www.tandfonline.com/action/journalInformation?journalCode=tjsp20 Journal of Systematic Palaeontology, 2019 Vol. 17, No. 12, 997–1029, http://dx.doi.org/10.1080/14772019.2018.1493547 Diversity and homologies of corystosperm seed-bearing structures from the Early Cretaceous of Mongolia aà b,c d e f Gongle Shi , Peter R. Crane , Patrick S. Herendeen , Niiden Ichinnorov , Masamichi Takahashi and Fabiany Herrerad aState Key Laboratory of Palaeobiology and Stratigraphy, Nanjing Institute of Geology and Palaeontology and Center for Excellence in Life and Paleoenvironment, Chinese Academy of Sciences, Nanjing 210008, China; bOak Spring Garden -
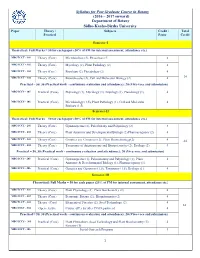
Syllabus for Post Graduate Course in Botany (2016 – 2017 Onward)
Syllabus for Post Graduate Course in Botany (2016 – 2017 onward) Department of Botany Sidho-Kanho-Birsha University Paper Theory / Subjects Credit / Total Practical Paper Credit Semester-I Theoretical: Full Marks = 50 for each paper (20% of FM for internal assessment, attendance etc.) MBOTCCT - 101 Theory (Core) Microbiology (2), Phycology (2) 4 MBOTCCT - 102 Theory (Core) Mycology (2), Plant Pathology (2) 4 MBOTCCT - 103 Theory (Core) Bryology (2), Pteridology (2) 4 MBOTCCT - 104 Theory (Core) Biomolecules (2), Cell and Molecular Biology (2) 4 24 Practical = 50, 30 (Practical work - continuous evaluation and attendance); 20 (Viva-voce and submission) MBOTCCS - 105 Practical (Core) Phycology (1), Mycology (1), Bryology (1), Pteridology (1). 4 MBOTCCS - 106 Practical (Core) Microbiology (1.5), Plant Pathology (1), Cell and Molecular 4 Biology (1.5). Semester-II Theoretical: Full Marks = 50 for each paper (20% of FM for internal assessment, attendance etc.) MBOTCCT - 201 Theory (Core) Gymnosperms (2), Paleobotany and Palynology (2) 4 MBOTCCT - 202 Theory (Core) Plant Anatomy and Developmental Biology (2) Pharmacognosy (2) 4 MBOTCCT - 203 Theory (Core) Genetics and Genomics (2), Plant Biotechnology(2) 4 24 MBOTCCT - 204 Theory (Core) Taxonomy of Angiosperms and Biosystematics (2), Ecology (2) 4 Practical = 50, 30 (Practical work - continuous evaluation and attendance); 20 (Viva-voce and submission) MBOTCCS - 205 Practical (Core) Gymnosperms (1), Palaeobotany and Palynology (1), Plant 4 Anatomy & Developmental Biology (1), Pharmacognosy (1). MBOTCCS - 206 Practical (Core) Genetics and Genomics (1.5), Taxonomy (1.5), Ecology (1). 4 Semester-III Theoretical: Full Marks = 50 for each paper (20% of FM for internal assessment, attendance etc.) MBOTCCT - 301 Theory (Core) Plant Physiology (2), Plant Biochemistry (2) 4 MBOTCCT - 302 Theory (Core) Economic Botany (2), Bioinformatics (2) 4 MBOTCCT - 303 Theory (Core) Elements of Forestry (2), Seed Technology (2). -

Evolução Das Plantas
EVOLUÇÃO DAS PLANTAS DAS EVOLUÇÃO Esta viagem pela Terra, pela sua formação, pelas suas primeiras atmosferas O segundo volume da coleção «Botânica em e vidas, pela evolução das plantas através das sucessivas mudanças é uma Português» faz uma síntese da história evolutiva leitura fascinante, às vezes difícil, mas como é mostrada e explicada com das plantas, desde a evolução da vida celular nas fontes grande sabedoria transmite conhecimento – saber científico –, que, apesar hidrotermais alcalinas oceânicas, há cerca de quatro EVOLUÇÃO das nossas falhas, conseguimos apreender e aprender. mil milhões de anos, até às grandes florestas tropicais hiperdiversas atuais. Como surgiu a fotossíntese? É uma lição de história, geologia, geografia, climatologia, agronomia As plantas nasceram na água: como invadiram a terra? e biologia, com notas de química e de física, cálculos matemáticos De que modo as plantas interagiram com a atmosfera DAS PLANTAS e paisagísticos, ou seja, a completa Aula de Botânica. terrestre? O que é e qual a origem do solo? Quais as funções das flores, esporos e sementes? Por que razão Carlos Aguiar Neste livro, desde a Pangeia, com a separação dos continentes, até hoje, as plantas com flor são tão bem-sucedidas? De que passando pelas várias erupções, avanços e recuos do mar, degelos e modo as megaextinções influenciaram a evolução das aquecimentos globais, vamos acompanhando os diferentes habitats, a | plantas? Estaremos perante uma nova megaextinção? Carlos Aguiar evolução e transformação das plantas pelos diversos continentes e mares Estas e muitas outras perguntas são respondidas – por seleção natural ou deriva genética – e como se foram aclimatando, ao longo deste livro. -

Assessing the Record and Causes of Late Triassic Extinctions
Earth-Science Reviews 65 (2004) 103–139 www.elsevier.com/locate/earscirev Assessing the record and causes of Late Triassic extinctions L.H. Tannera,*, S.G. Lucasb, M.G. Chapmanc a Departments of Geography and Geoscience, Bloomsburg University, Bloomsburg, PA 17815, USA b New Mexico Museum of Natural History, 1801 Mountain Rd. N.W., Albuquerque, NM 87104, USA c Astrogeology Team, U.S. Geological Survey, 2255 N. Gemini Rd., Flagstaff, AZ 86001, USA Abstract Accelerated biotic turnover during the Late Triassic has led to the perception of an end-Triassic mass extinction event, now regarded as one of the ‘‘big five’’ extinctions. Close examination of the fossil record reveals that many groups thought to be affected severely by this event, such as ammonoids, bivalves and conodonts, instead were in decline throughout the Late Triassic, and that other groups were relatively unaffected or subject to only regional effects. Explanations for the biotic turnover have included both gradualistic and catastrophic mechanisms. Regression during the Rhaetian, with consequent habitat loss, is compatible with the disappearance of some marine faunal groups, but may be regional, not global in scale, and cannot explain apparent synchronous decline in the terrestrial realm. Gradual, widespread aridification of the Pangaean supercontinent could explain a decline in terrestrial diversity during the Late Triassic. Although evidence for an impact precisely at the boundary is lacking, the presence of impact structures with Late Triassic ages suggests the possibility of bolide impact-induced environmental degradation prior to the end-Triassic. Widespread eruptions of flood basalts of the Central Atlantic Magmatic Province (CAMP) were synchronous with or slightly postdate the system boundary; emissions of CO2 and SO2 during these eruptions were substantial, but the contradictory evidence for the environmental effects of outgassing of these lavas remains to be resolved. -

Fossil and Living Cycads Say No More Megasporophylls
hology orp a Miao et al., J Morphol Anat 2017, 1:2 nd M f A o n l a a t n o r m u y o J Journal of Morphology and Anatomy Research Article Article Open Access Fossil and Living Cycads Say "No More Megasporophylls" Yuyan Miao1,2, Zhong-Jian Liu3, Meina Wang3,4 and Xin Wang5* 1Beijing Museum of Natural History, Beijing, China 2State Key Laboratory of Biogeology and Environmental Geology, China University of Geosciences (Wuhan), Wuhan, China 3Shenzhen Key Laboratory for Orchid Conservation and Utilization, National Orchid Conservation Center of China and Orchid Conservation and Research Center of Shenzhen, Shenzhen, China 4College of Landscape Architecture, Fujian Agriculture and Forestry University, Fuzhou, China 5CAS Key Laboratory of Economic Stratigraphy and Paleogeography, Nanjing Institute of Geology and Palaeontology, Nanjing, China Abstract The origins of angiosperms and cycads are still mysterious. To understand the evolution of these groups as well as other gymnosperms it was impossible without mentioning a frequently used term “megasporophyll”. “Megasporophyll” is a concept that has been used widely in botany. This term is more or less related with the famous saying “Alles ist Blatt” by Goethe. This term became popular since Arber and Parkin hypothesized that the carpels in the Magnoliales were equivalent to and derived from former foliar parts bearing ovules along their margins (“megasporophyll”). Many botanists uncritically called the parts in all the reproductive organs of seed plants as “sporophylls”, no matter what they actually saw in the plants. However, the fact is that none of the reproductive parts (fossil or living), except those in the Cycadales, are foliar or leaf-like.