Specimen Labeling and Handling
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Food Packaging (FS 522 / FS 495) Aseptic Processing and Packaging
Food Packaging (FS 522 / FS 495) Aseptic Processing and Packaging Components of an aseptic processing system Aseptic processing is a thermal process in which the product and container are sterilized separately and brought together in a sterile environment. It involves pumping, deaeration, and sterilization of a food product, followed by holding it for a specified period of time (in a holding tube -- required to have a 1/4" rise per foot length of tube), cooling it, and finally packaging it in a sterile container. The use of high temperature for a short period of time (in comparison with conventional canning) in aseptic processing yields a high quality product. Care should be taken to ensure that all process calculations are performed after the deaeration stage and not based on the initial raw product. Deaeration is accomplished in a vessel maintained at a certain degree of vacuum by means of a vacuum pump. The product is fed into the vessel at 55 - 70 /C through a nozzle at the center of the vessel. Vacuum is controlled to obtain a product flash of about 5 /C. An internal spiral condenser condenses vapors and other condensable gases. The deaerated product is discharged through the bottom and pumped to the heating section. Another important part of an aseptic processing system is the back pressure valve which provides sufficient pressure to prevent boiling of the product at processing temperatures which can be as high as 125-130 /C. An aseptic surge tank provides the means for product to be continuously processed even if the packaging system is not operational due to any malfunction. -

Gas Generator Bottle Introduction SCIENTIFIC This Gas Generator Setup Provides an Easy Way to Generate and Collect Gas
Gas Generator Bottle Introduction SCIENTIFIC This gas generator setup provides an easy way to generate and collect gas. Specific instructions are provided for the generation of hydrogen gas using zinc and acid. Concepts • Generation of gases • Water displacement Materials Hydrochloric acid solution, HCl, 3 M Glass plates or Sulfuric acid solution, H2SO4, 3 M Glass tubing Mossy zinc, Zn, 6 g Pneumatic trough Water, tap Rubber tubing Bent glass tubing* Silicone grease packet* Gas collecting bottles or tubes, 3 or 4 Thistle tube* Gas generator bottle* Two-hole rubber stopper* *Materials included. Safety Precautions Hydrochloric acid solution is toxic by ingestion and inhalation and is severely corrosive to skin, eyes and other tissues, as is sulfuric acid solu- tion. Hydrogen gas is a highly flammable gas and a severe fire hazard. Exercise extreme caution when testing the gas and keep the gas generator away from flames. Wear chemical splash goggles, chemical-resistant gloves, and a chemical-resistant apron. This activity requires the use of hazardous components and/or has the potential for hazardous reactions. Please review current Material Safety Data Sheets for additional safety, handling, and disposal information. Procedure 1. Set up the apparatus as shown in the figure to the right. Lubricate the glass tubing and thistle tube with silicone grease before inserting into the stopper. Make sure Thistle tube the water level is above the platform. Prepare bottles for collecting gas by water Two-hole rubber stopper displacement. To do this, fill each gas collecting bottle (or tube) over the brim with tap water, and then cover each with a flat glass plate. -

Trace Elements Specimen Collection Guide
500 Chipeta Way Salt Lake City, Utah 84108-1221 Phone: 801-583-2787 | toll free: 800-242-2787 A nonprofit enterprise of the University of Utah and its Department of Pathology Fax: 801-522-2706 | aruplab.com TRACE ELEMENTS SPECIMEN COLLECTION GUIDE Analysis for trace elements at ARUP is performed in a clean laboratory environment that includes a system of positive- pressure and HEPA-filtered air. Since detection limits for many trace elements are calculated in parts per billion, this helps minimize environmental contamination of specimens. Additionally, contamination control during specimen collection provides more accurate and clinically useful results. Note: Refer to the ARUP Laboratory Test Directory for specific instructions when collecting specimens for cadmium exposure testing (http://ltd.aruplab.com/Tests/Pub/0025013). Urine Collection Tissue Although it is nearly impossible to obtain a completely Follow information on individual pages of the laboratory uncontaminated urine specimen, steps can be taken to test directory. minimize environmental contamination. Avoid collecting the specimen in an area where Whole Blood, Serum, and Red Blood Cell Collection Materials comprising the tube and the tube top are a environmental contamination is likely to occur. In an consideration. Rubber stoppers in collection tubes other industrial or construction setting, it is important that than the royal blue tube are known to contain elements clothing worn in the workplace be removed prior to that can contaminate a specimen. Contamination can be specimen collection to prevent dust on the clothing introduced as the needle punctures the stopper in a tube from contaminating the specimen. other than royal blue. Most of this also holds true for Wash and dry hands thoroughly prior to collection. -

Rigid Intermediate Bulk Containers: United States
Freedonia Focus Reports US Collection Rigid Intermediate Bulk Containers: United States February 2019 CLICK TO ORDER FULL REPORT BROCHURE www.freedoniafocusreports.com CLICK TO ORDER FULL REPORT Table of Contents 1. Highlights 3 2. Market Environment 4 Historical Trends 4 Key Economic Indicators 5 Competition from Reconditioned & Refurbished Packaging 6 Regulatory Factors 8 3. Segmentation & Forecasts 10 Products 10 Plastic Body 11 Metal Body 12 Other Rigid Intermediate Bulk Containers 13 Markets 14 Chemicals & Pharmaceuticals 15 Food & Beverages 15 Petroleum & Lubricants 16 Hazardous Waste 16 Agricultural & Horticultural Products 16 Plastic & Rubber 17 Other Markets 17 4. Industry Structure 19 Industry Characteristics 19 Market Leaders 20 Mauser Packaging Solutions 20 SCHÜTZ 21 Greif 21 5. About This Report 22 Scope 22 Sources 22 Industry Codes 23 Freedonia Methodology 23 Resources 25 Rigid Intermediate Bulk Containers: United States 1 ©2019 The Freedonia Group. All rights reserved. List of Tables & Figures Figure 1 | Key Trends in the US Rigid Intermediate Bulk Container Market, 2018 – 2023 3 Figure 2 | US Rigid Intermediate Bulk Container Demand Trends, 2008 – 2018 4 Table 1 | Key Indicators for US Rigid Intermediate Bulk Container Demand, 2008 – 2023 (US$ bil) 5 Figure 3 | US Rigid Intermediate Bulk Container Demand by Product, 2008 – 2023 (US$ mil) 10 Table 2 | US Rigid Intermediate Bulk Container Demand by Product, 2008 – 2023 (US$ mil) 10 Figure 4 | US Rigid Intermediate Bulk Container Demand by Product, 2008 – 2023 (%) 12 Figure 5 | US Rigid Intermediate Bulk Container Demand by Market, 2008 – 2023 (US$ mil) 14 Table 3 | US Rigid Intermediate Bulk Container Demand by Market, 2008 – 2023 (US$ mil) 14 Figure 6 | US Rigid Intermediate Bulk Container Demand by Market, 2008 – 2023 (%) 18 Table 4 | Leading Suppliers in the US Rigid Intermediate Bulk Container Market by Product 20 Table 5 | NAICS & SIC Codes Related to Rigid Intermediate Bulk Containers 23 Rigid Intermediate Bulk Containers: United States 2 ©2019 The Freedonia Group. -

Nalgene and Nunc Centrifuge Ware Catalog
Nalgene and Nunc Centrifuge Ware Select the right vessel and spin with confidence Spin with confidence at virtually any scale The process of selecting a centrifuge and rotor can feel like the easy part when faced with choosing the tube or bottle that is the right fit for both the rotor and application. There are several factors to consider when selecting the correct vessel for each application: • Chemical compatibility • Volume • Temperature • Relative centrifugal force (RCF) required • Protocols to be used for loading and sample recovery • Cleaning and autoclaving steps Understanding your requirements before selecting a tube or bottle ensures you make the right choice. Whether your application includes the need for separations, large volume pelleting, protein purification or DNA isolation, the comprehensive selection of Thermo Scientific™ Nalgene™ and Thermo Scientific™ Nunc™ centrifuge ware offers a solution for virtually scales and is available in sizes from 10 mL to 2 L. Such a broad offering means a tube or bottle for many spins – from clinical and bioproduction, to processing bacteria, yeast, tissue, and viruses. Contents Centrifuge tubes 4 Centrifuge bottles 20 Closures and adaptors 31 Resources 36 Simplify performance at every turn with a reliable and safe approach to centrifugation Nalgene Conical-Bottom Centrifuge Tubes polypropylene copolymer Thermo Scientific™ Nalgene™ PPCO conical-bottom centrifuge tubes with molded-in graduations have excellent chemical resistance. Designed for low-speed centrifugation in refrigerated and non-refrigerated centrifuges details • Translucent PPCO is compatible with a wide range of lab reagents • Conical bottoms concentrate pellet in a small area for easy isolation and retrieval • Molded-in graduations last the life of the tube • Last longer than polycarbonate tubes under conditions of repeated Note: Centrifuge tubes must be filled at least 80% for proper performance. -
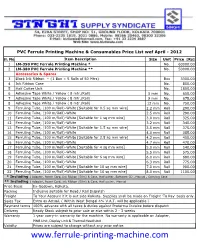
Ferrule Printing Machine & Consumables Price List Wef April - 2012
PVC Ferrule Printing Machine & Consumables Price List wef April - 2012 Sl. No. Item Description Size Unit Price [Rs] 1 LM-390 PVC Ferrule Printing Machine * No. 60000.00 2 LM-380 PVC Ferrule Printing Machine ** No. 50000.00 Accessories & Spares 3 Black Ink Ribbon – {1 Box = 5 Rolls of 50 Mtrs} Box 3300.00 4 Ink Ribbon Case No. 850.00 5 Half Cutter Unit No. 1800.00 6 Adhesive Tape White / Yellow ( 8 mtr./Roll) 5 mm No. 650.00 7 Adhesive Tape White / Yellow ( 8 mtr./Roll) 9 mm No. 675.00 8 Adhesive Tape White / Yellow ( 8 mtr./Roll) 12 mm No. 750.00 9 Ferruling Tube, (100 m/Roll)-White [Suitable for 0.5 sq mm wire] 2.2 mm Roll 290.00 10 Ferruling Tube, (100 m/Roll)-White 2.5 mm Roll 290.00 11 Ferruling Tube, (100 m/Roll)-White [Suitable for 1 sq mm wire] 3.0 mm Roll 325.00 12 Ferruling Tube, (100 m/Roll)-White 3.2 mm Roll 340.00 13 Ferruling Tube, (100 m/Roll)-White [Suitable for 1.5 sq mm wire] 3.5 mm Roll 375.00 14 Ferruling Tube, (100 m/Roll)-White 4.0 mm Roll 385.00 15 Ferruling Tube, (100 m/Roll)-White [Suitable for 2.5 sq mm wire] 4.2 mm Roll 450.00 16 Ferruling Tube, (100 m/Roll)-White 4.7 mm Roll 470.00 17 Ferruling Tube, (100 m/Roll)-White [Suitable for 4 sq mm wire] 5.0 mm Roll 540.00 18 Ferruling Tube, (100 m/Roll)-White 5.5 mm Roll 575.00 19 Ferruling Tube, (100 m/Roll)-White [Suitable for 6 sq mm wire] 6.0 mm Roll 680.00 20 Ferruling Tube, (100 m/Roll)-White 6.3 mm Roll 775.00 21 Ferruling Tube, (100 m/Roll)-White [Suitable for 10 sq mm wire] 7.0 mm Roll 900.00 22 Ferruling Tube, (100 m/Roll)-White [Suitable for 16 sq mm wire] 8.0 mm Roll 1100.00 * Including : Adapter, Power Cord, Ink Ribbon (50m) & Case, Half cutter, Software CD , Manual , Carrying Case ** Including : Adapter, Power Cord, Ink Ribbon (50m) & Case, Half cutter, Manual Price Basis Ex-Godown, Kolkata. -

Urinary Drainage Bottle Urine Collection System
URINARY DRAINAGE BOTTLE URINE COLLECTION SYSTEM FOR USE WITH FOLEY INDWELLING CATHETERS, MALE EXTERNAL CATHETERS AND UROSTOMY APPLICATIONS USE AND CARE INSTRUCTIONS FOR PRODUCT #4100 GH-F HI LO us W o 13 L i m t a t F l e r R u e o d s V e r a l u 410002 v t e a ™ e F LATEX-FREE DEHP-FREE KIT CONTENTS: 1 Durable Urinary Drainage Bottle (#410001) 1 Bottle Cap with Housing (#4101) 1 60" (1.5m) Drainage Tube with Adaptor (#6061) 1 Adjustable Tube Clamp (#6999) 1 Urinary Drainage Bottle Adaptor (#6003) 1 “HIGH-FLOW” Red Anti-Reflux Valve (#6001) 1 Large Gasket-Ring (#6000) 1 Pair of Hooks for Hanging (#4102) 1 Illustrated Use and Care Instructions CAUTION: If red anti-reflux valve is missing or removed, leaks around the top valve housing may occur. The use of Gasket-Ring (#6000) will be necessary to seal the housing threads and prevent leaking. #6003 Urinary Drainage Bottle Adaptor #6061 Clear-Vinyl Drainage Tube with Graduated Adaptor and Cap # 6000 Black Gasket-Ring # replace for anti-reflux valve 13 6001 High-Flow #6999 Adjustable when not in use. Anti-Reflux Valve Thumb Clamp IMPORTANT: To avoid drainage problems, before using this product, read all instructions #6003 Urinary Drainage and information carefully. 13 Bottle Adaptor Understand how it works and how to maintain it properly. #6001 High-Flow #4101 Anti-Reflux Valve 13 Urinary Drainage FIGURE 2 Bottle Cap #4101 TOP VALVE HOUSING DETAIL Urinary Drainage Bottle Cap #6003 Urinary Drainage Bottle Adaptor #603005 Top Valve Housing (not sold separately) #4102 Urinary Drainage Bottle Hooks # 410001 Drainage Bottle (not sold separately) 410002 #410001 Urinary Drainage Bottle (not sold separately) FIGURE 3 BOTTLE CAP CLOSE-UP To remove adaptor (#6003) from bottle cap, remove tubing (#6061), hold housing (#603005) in place and turn adaptor counter-clockwise. -

Package Codes
PACKAGE CODES CODE DESCRIPTION 43 Bag, super bulk 1A Drum, steel 1B Drum, aluminium 1D Drum, plywood 1G Drum, fibre 1W Drum, wooden 2C Barrel, wooden 3A Jerry-can, steel 3H Jerry-can, plastic 4A Box, steel 4B Box, aluminum 4C Box, natural wood 4D Box, plywood 4F Box, reconstituted wood 4G Box, fibreboard 4H Box, plastic 5H Bag, woven plastic 5L Bag, textile 5M Bag, paper 6H ComposIte packaging, plastic receptable 6P Composite packaging, glass receptaple AA Intermediate bulk container, rigid plastic AB Receptable, fibre AC Receptable, paper AD Receptable, wooden AE Aerosol AF Pallet, modular, collars 80cm x 60cm AG Pallet, shrink-wrapped AH Pallet, 100 cm x 110cm AI Clamshell AJ Cone AM Ampoule, non-protected AP Ampoule, protected AT Atomiser AV Capsule BA Barrel BB Bobbin BC Bottlecrate, bottlerack BD Board BE Bundle BF Balloon, non-protected BG Bag BH Bunch BI Bin BJ Bucket BK Basket BL Bale, compressed BM Basin Page 1 of 8 PACKAGE CODES CODE DESCRIPTION BN Bale, non-compressed BO Bottle, non-protected, cylindrical BP Balloon, protected BQ Bottle, protected cylindrical BR Bar BS Bottle, non-protected, bulbous BT Bolt BU Butt BV Bottle, protected bulbous BW Box, for liquids BX Box BY Board, in bundle/bunch/truss, BZ Bars, in bundle/bunch/truss CA Can, rectangular CB Beer crate CC Churn CD Can, with handle and spout CE Creel CF Coffer CG Cage CH Chest CI Canister CJ Coffin CK Cask CL Coil CM Collis CN Container not otherwise specified as transport equipment CO Carboy, non-protected CP Carboy, protected CQ Cartdidge CR Crate CS Case CT -

Guidelines for Unit Packing for Integrated Circuits - Tubes (Rails)
Guidelines for Unit Packing for Integrated Circuits - Tubes (Rails) NIGP 102.00 SEPTEMBER 1992 NATIONAL ELECTRONIC DISTRIBUTORS ASSOCIATION 1111 Alderman Drive, Suite 400 Alpharetta, GA 30005-4143 678-393-9990/678-393-9998 fax www.nedassoc.org NOTICE NEDA Industry Guidelines and Publications contain material that has been prepared, progressively reviewed, and approved through various NEDA sponsored industry task forces comprised of NEDA member distributors and manufacturers and subsequently reviewed and approved by the NEDA Board of Directors. NEDA Industry Guidelines and Publications are designed to serve the public interest including electronic component distributors through the promotion of standardized practices between manufacturers and distributors resulting in improved efficiency, profitability and product quality. Existence of such guidelines shall not in any respect preclude any member or nonmember of NEDA from selling or manufacturing products not in conformance to such guidelines, nor shall the existence of such guidelines preclude their voluntary use by those other than NEDA members, whether the guideline is to be used either domestically or internationally. NEDA does not assume any liability or obligation whatever to parties adopting NEDA industry Guidelines and Publications. Inquiries, comments and suggestions relative to the content of this NEDA Industry Guideline should be addressed to NEDA headquarters. Published by NATIONAL ELECTRONIC DISTRIBUTORS ASSOCIATION 1111 Alderman Drive, Suite 400 Alpharetta, GA 30005 678.393.9990/678.393.9998 fax Copyright 1992 Printed in U.S.A. All rights reserved NEDA Guidelines for Unit Packing for Integrated Circuits - Tubes (Rails) Introduction Growing global competition for the industries which comprise the predominant users of electronic components, particularly semiconductor products, is resulting in a continuous evolution in customers' practices and their vendor performance expectations. -

Smart Toothpaste Dispenser Design Document
Smart Toothpaste Dispenser Design Document Jianqiao Xiao, Zhaoying Du, Yusheng Wei Spring 2019 1. Introduction 1.1 Objective When people manually squeeze toothpaste from the tube, they tend to overdose on toothpaste and experience difficulty getting the last。 According to a study of the rational use of fluoride toothpaste, a child should use a pea size (0.25 grams) of toothpaste and an adult should use about 0.5 grams of toothpaste each time for the best effect [1]. However, many people cover the entire brush with toothpaste for each use, which results in using 1~1.5 grams of toothpaste [2], largely exceeding the optimum amount. In addition, when most of the toothpaste in the tube is used, users may need to roll the tube up or use a toothpaste squeezer to get the rest out. This process is usually annoying, and much toothpaste may get wasted. Because of the tendency of overdosing and wasting toothpaste, we propose to design an electric toothpaste dispenser, which can accurately control the amount of toothpaste dispensed each time and prevent toothpaste overdosing. With a peristaltic pump installed, the dispenser effectively gets the toothpaste out of the tube and reduce the wasted toothpaste. 1.2 Background The majority of the toothpaste dispensers in the market are manual. After purchasing one existing model of toothpaste dispenser and disassembling it, we discovered that a mechanical manual pump was used to suck toothpaste from the tube. The manual pump consists of a silicone container with two silicone one-way control valves. When the rubber container is squeezed, the toothpaste buffered in the container goes out to the user, and when the container is released, the elasticity of the container creates suction and pumps the toothpaste out of the tube. -

How Single-Use Connections Advance Aseptic Processing: Increased Process Flexibility and Reliability, Reduced Costs
WHITE PAPER 7004 How single-use connections advance aseptic processing: Increased process flexibility and reliability, reduced costs By Today’s market demand on biologic and The connecting device or connection John Boehm pharmaceutical manufacturers to develop new method may appear to be a small part Vice President of Biopharma drugs — combined with the economic environment of an overall system, however, connection Colder Products Company — is challenging bioprocessors to review their and disconnection of tubing for process processing systems and seek ways to make fluid transfer is a critical aspect of single- them more flexible, reliable and cost effective. use processing. Manufacturers need to Increasingly, manufacturers are turning to carefully consider the available options single-use aseptic processing systems to meet or because the connector can be the deciding beat aggressive product-introduction time frames factor in keeping the single-use bioprocess and control costs. truly aseptic. Innovative single-use technologies provide STERILE CONNECTION OPTIONS pharmaceutical manufacturers greater flexibility for replacing traditional stainless There are many ways by which tubing, equipment or even entire process biopharmaceutical manufacturers suites with plastic-based processing can create a sterile connection within or solutions. The benefits of converting to between their unit operations. The right pre-sterilized single-use systems have been selection is highly dependent on the needs documented in numerous articles and case and preferences of each facility. studies, and these benefits would be lost if manufacturers could not safely and securely Quick disconnect couplings or fittings connect the various pieces of equipment to (sanitary and luer) are commonly used with create a complete aseptic process. -

Evidence Packaging: a How-To Guide
Evidence Packaging: A How-To Guide Terry Spear, John Rush, Jerry Massetti, Jim Weigand and Mark Traughber California Department of Justice Bureau of Forensic Services Goals of Evidence Packaging Protects personnel from possible hazards associated with evidence: Accidental firearm discharge Biohazards Sharp objects Health hazards associated with exposure to mold • Wet plant material Goals of Evidence Packaging Protects evidence against: Loss Contamination Cross-transfer • Suspect to victim • Victim to suspect • Scene to scene • Item to item Deterioration Goals of Evidence Packaging Correctly identifies evidence with respect to: Evidence description: What is it? Evidence source • Origin – where was evidence when it was collected • Custodial Agency Chain of custody Provides a unique identifier so it cannot be confused with any other evidence item • Laboratory case and item number Packaging Can indicate What processing is needed • For example: “Fingerprint processing required” Health hazards (e.g. sharp knife) Required or preferred storage conditions • “Store at room temperature” • “Store frozen” Elements of packaging evidence Does evidence need to be prepared for packaging? Certain types of evidence may need to be dried before it can be packaged: • Wet biological samples • Fresh plant material What Can Happen When Biological Evidence is Packaged Wet? Evidence “leaks” through to the exterior of the container Elements of packaging evidence What type of packaging is optimal? Plastic: What type of plastic? • Envelope •