1 Students Will Create a Cartesian Diver, Which Will Act in Some Ways
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Food Packaging (FS 522 / FS 495) Aseptic Processing and Packaging
Food Packaging (FS 522 / FS 495) Aseptic Processing and Packaging Components of an aseptic processing system Aseptic processing is a thermal process in which the product and container are sterilized separately and brought together in a sterile environment. It involves pumping, deaeration, and sterilization of a food product, followed by holding it for a specified period of time (in a holding tube -- required to have a 1/4" rise per foot length of tube), cooling it, and finally packaging it in a sterile container. The use of high temperature for a short period of time (in comparison with conventional canning) in aseptic processing yields a high quality product. Care should be taken to ensure that all process calculations are performed after the deaeration stage and not based on the initial raw product. Deaeration is accomplished in a vessel maintained at a certain degree of vacuum by means of a vacuum pump. The product is fed into the vessel at 55 - 70 /C through a nozzle at the center of the vessel. Vacuum is controlled to obtain a product flash of about 5 /C. An internal spiral condenser condenses vapors and other condensable gases. The deaerated product is discharged through the bottom and pumped to the heating section. Another important part of an aseptic processing system is the back pressure valve which provides sufficient pressure to prevent boiling of the product at processing temperatures which can be as high as 125-130 /C. An aseptic surge tank provides the means for product to be continuously processed even if the packaging system is not operational due to any malfunction. -

Determination of the Identity of an Unknown Liquid Group # My Name the Date My Period Partner #1 Name Partner #2 Name
Determination of the Identity of an unknown liquid Group # My Name The date My period Partner #1 name Partner #2 name Purpose: The purpose of this lab is to determine the identity of an unknown liquid by measuring its density, melting point, boiling point, and solubility in both water and alcohol, and then comparing the results to the values for known substances. Procedure: 1) Density determination Obtain a 10mL sample of the unknown liquid using a graduated cylinder Determine the mass of the 10mL sample Save the sample for further use 2) Melting point determination Set up an ice bath using a 600mL beaker Obtain a ~5mL sample of the unknown liquid in a clean dry test tube Place a thermometer in the test tube with the sample Place the test tube in the ice water bath Watch for signs of crystallization, noting the temperature of the sample when it occurs Save the sample for further use 3) Boiling point determination Set up a hot water bath using a 250mL beaker Begin heating the water in the beaker Obtain a ~10mL sample of the unknown in a clean, dry test tube Add a boiling stone to the test tube with the unknown Open the computer interface software, using a graph and digit display Place the temperature sensor in the test tube so it is in the unknown liquid Record the temperature of the sample in the test tube using the computer interface Watch for signs of boiling, noting the temperature of the unknown Dispose of the sample in the assigned waste container 4) Solubility determination Obtain two small (~1mL) samples of the unknown in two small test tubes Add an equal amount of deionized into one of the samples Add an equal amount of ethanol into the other Mix both samples thoroughly Compare the samples for solubility Dispose of the samples in the assigned waste container Observations: The unknown is a clear, colorless liquid. -

Acton Liquor Store
1966 Experience The Colonial Difference02 Holiday Spirits from Around the World! 02 Special Savings on Our original location Beer and Wine! 04 Add Some Sparkle: in 1966 Champagne Deals! 07 How Much of What? Party Planning from Colonial Spirits the Experts 08 Come celebrate our 46th season Wine Matches for Every Family Favorite! 010 Serve Wine Like a Sommelier! 015 Beer and Food, Done Right! 016 Moonshine North of the Mason-Dixon! 019 Finding Your Way in Whisk[e]y! 022 The New (Old) Wine Fashions! 024 4th Annual Big Red Tasting 026 Touching the Roots of Wine: Blends! 028 2012 87 Great Road, Acton, MA 01720 978.263.7775 Order Online at: www.ColonialSpiritsDelivers.com Colonial Spirits began its service as a but its popularity and the choices available to enthusiasts developed wine shop to the Acton, Concord, Carlisle and surrounding communi- quickly. Colonial Spirits went through several expansions over the ties over 40 years ago. Along a lightly developed and traveled route years to keep up with the ever growing demand for selection and the 2A in East Acton, Colonial Spirits began in the 19th century building diverse and changing tastes of people in the community. Wine proved next to the street. In its early days Colonial Spirits’ selection would to be a major source of enjoyment for people as new wineries from all seem quite limited in comparison to what can be found in the shop over the world continued to become available in Colonial Spirits. What today. Wine was just beginning to become a major consumer product, is most prominent in our recent history is the time spent at 69 Great Rd and the major expansion into 87 Great Rd in 2003. -

Gas Generator Bottle Introduction SCIENTIFIC This Gas Generator Setup Provides an Easy Way to Generate and Collect Gas
Gas Generator Bottle Introduction SCIENTIFIC This gas generator setup provides an easy way to generate and collect gas. Specific instructions are provided for the generation of hydrogen gas using zinc and acid. Concepts • Generation of gases • Water displacement Materials Hydrochloric acid solution, HCl, 3 M Glass plates or Sulfuric acid solution, H2SO4, 3 M Glass tubing Mossy zinc, Zn, 6 g Pneumatic trough Water, tap Rubber tubing Bent glass tubing* Silicone grease packet* Gas collecting bottles or tubes, 3 or 4 Thistle tube* Gas generator bottle* Two-hole rubber stopper* *Materials included. Safety Precautions Hydrochloric acid solution is toxic by ingestion and inhalation and is severely corrosive to skin, eyes and other tissues, as is sulfuric acid solu- tion. Hydrogen gas is a highly flammable gas and a severe fire hazard. Exercise extreme caution when testing the gas and keep the gas generator away from flames. Wear chemical splash goggles, chemical-resistant gloves, and a chemical-resistant apron. This activity requires the use of hazardous components and/or has the potential for hazardous reactions. Please review current Material Safety Data Sheets for additional safety, handling, and disposal information. Procedure 1. Set up the apparatus as shown in the figure to the right. Lubricate the glass tubing and thistle tube with silicone grease before inserting into the stopper. Make sure Thistle tube the water level is above the platform. Prepare bottles for collecting gas by water Two-hole rubber stopper displacement. To do this, fill each gas collecting bottle (or tube) over the brim with tap water, and then cover each with a flat glass plate. -

How Does the Diver Work? Preparing the Plastic Soda Bottle
How Does the Diver Work? Preparing the Plastic Soda Bottle Vv'hen you build a Cartesian diver, you are exploring three scientific properties of air: You will need to start collecting plastic soda bottles with caps. While (1) Air has weight almost any size bottle will work, the most popular sizes are 1 liter, 1.5 liter, and 2 liter bottles. Smaller children will find that the 1 and 1.5 liter (2) Air occupies space bottles are easiest to squeeze. The best soda bottles are those that are (3) Air exerts pressure. clear from top to bottom so that you can see everything that is happening in the bottle. Generally speaking, an object will float in a fluid if its density is less than that of the fluid (densltyemass/volume). If the object is more dense than the fluid, then the object will sink. For example, an empty bottle will float in a bathtub that is filled with water if the bottle is less dense than the water. However, as you start filling the bottle with water, its Here's an easy method for density increases and its buoyancy decreases. Eventually, the bottle will sink if it is filled too full with water. ~ cleaning the plastic The Cartesian diver, consisting of a plastic medicine dropper and soda bottles: a metal hex nut, will float or sink in the bottle of water depending on the water level in the bulb of the dropper. Vv'hen pressure is applied to the outside of the bottle, water is pushed up inside the diver, and the air • Rinse out the bottle using warm water. -

Trace Elements Specimen Collection Guide
500 Chipeta Way Salt Lake City, Utah 84108-1221 Phone: 801-583-2787 | toll free: 800-242-2787 A nonprofit enterprise of the University of Utah and its Department of Pathology Fax: 801-522-2706 | aruplab.com TRACE ELEMENTS SPECIMEN COLLECTION GUIDE Analysis for trace elements at ARUP is performed in a clean laboratory environment that includes a system of positive- pressure and HEPA-filtered air. Since detection limits for many trace elements are calculated in parts per billion, this helps minimize environmental contamination of specimens. Additionally, contamination control during specimen collection provides more accurate and clinically useful results. Note: Refer to the ARUP Laboratory Test Directory for specific instructions when collecting specimens for cadmium exposure testing (http://ltd.aruplab.com/Tests/Pub/0025013). Urine Collection Tissue Although it is nearly impossible to obtain a completely Follow information on individual pages of the laboratory uncontaminated urine specimen, steps can be taken to test directory. minimize environmental contamination. Avoid collecting the specimen in an area where Whole Blood, Serum, and Red Blood Cell Collection Materials comprising the tube and the tube top are a environmental contamination is likely to occur. In an consideration. Rubber stoppers in collection tubes other industrial or construction setting, it is important that than the royal blue tube are known to contain elements clothing worn in the workplace be removed prior to that can contaminate a specimen. Contamination can be specimen collection to prevent dust on the clothing introduced as the needle punctures the stopper in a tube from contaminating the specimen. other than royal blue. Most of this also holds true for Wash and dry hands thoroughly prior to collection. -

Laboratory Equipment Reference Sheet
Laboratory Equipment Stirring Rod: Reference Sheet: Iron Ring: Description: Glass rod. Uses: To stir combinations; To use in pouring liquids. Evaporating Dish: Description: Iron ring with a screw fastener; Several Sizes Uses: To fasten to the ring stand as a support for an apparatus Description: Porcelain dish. Buret Clamp/Test Tube Clamp: Uses: As a container for small amounts of liquids being evaporated. Glass Plate: Description: Metal clamp with a screw fastener, swivel and lock nut, adjusting screw, and a curved clamp. Uses: To hold an apparatus; May be fastened to a ring stand. Mortar and Pestle: Description: Thick glass. Uses: Many uses; Should not be heated Description: Heavy porcelain dish with a grinder. Watch Glass: Uses: To grind chemicals to a powder. Spatula: Description: Curved glass. Uses: May be used as a beaker cover; May be used in evaporating very small amounts of Description: Made of metal or porcelain. liquid. Uses: To transfer solid chemicals in weighing. Funnel: Triangular File: Description: Metal file with three cutting edges. Uses: To scratch glass or file. Rubber Connector: Description: Glass or plastic. Uses: To hold filter paper; May be used in pouring Description: Short length of tubing. Medicine Dropper: Uses: To connect parts of an apparatus. Pinch Clamp: Description: Glass tip with a rubber bulb. Uses: To transfer small amounts of liquid. Forceps: Description: Metal clamp with finger grips. Uses: To clamp a rubber connector. Test Tube Rack: Description: Metal Uses: To pick up or hold small objects. Beaker: Description: Rack; May be wood, metal, or plastic. Uses: To hold test tubes in an upright position. -

EARTH SCIENCE ACTIVITY #1 Tsunami in a Bottle
EARTH SCIENCE ACTIVITY #1 Grades 3 and Up Tsunami in a Bottle This activity is one of several in a basic curriculum designed to increase student knowledge about earthquake science and preparedness. The activities can be done at any time in the weeks leading up to the ShakeOut drill. Each activity can be used in classrooms, museums, and other educational settings. They are not sequence-bound, but when used together they provide an overview of earthquake information for children and students of various ages. All activities can be found at www.shakeout.org/schools/resources/. Please review the content background (page 3) to gain a full understanding of the material conducted in this activity. OBJECTIVE: For students to learn that tsunamis can be caused by earthquakes and to understand the effects of tsunamis on the shoreline MATERIALS/RESOURCES NEEDED: 2-liter plastic soda bottles Small gravel (fish tank gravel) Water source Empty water bottle (16 oz) Overhead projector Transparency of Tsunami Facts “What Do I See?” handout PRIOR KNOWLEDGE: In order to conduct this activity, students need to know how fault slippage can generate earthquakes. ACTIVITY: Set-Up (Time varies) Collect as many 2-liter soda bottles as possible or ask students to bring in bottles for this activity (3 students can share one bottle). Obtain an empty water bottle (about 16 oz). Remove labels from all bottles. Purchase or gather enough small gravel to fit through the mouth of the soda bottles. Students will fill up their soda bottles with gravel to create at least a 2 inch layer on the bottom of the bottle. -

Rigid Intermediate Bulk Containers: United States
Freedonia Focus Reports US Collection Rigid Intermediate Bulk Containers: United States February 2019 CLICK TO ORDER FULL REPORT BROCHURE www.freedoniafocusreports.com CLICK TO ORDER FULL REPORT Table of Contents 1. Highlights 3 2. Market Environment 4 Historical Trends 4 Key Economic Indicators 5 Competition from Reconditioned & Refurbished Packaging 6 Regulatory Factors 8 3. Segmentation & Forecasts 10 Products 10 Plastic Body 11 Metal Body 12 Other Rigid Intermediate Bulk Containers 13 Markets 14 Chemicals & Pharmaceuticals 15 Food & Beverages 15 Petroleum & Lubricants 16 Hazardous Waste 16 Agricultural & Horticultural Products 16 Plastic & Rubber 17 Other Markets 17 4. Industry Structure 19 Industry Characteristics 19 Market Leaders 20 Mauser Packaging Solutions 20 SCHÜTZ 21 Greif 21 5. About This Report 22 Scope 22 Sources 22 Industry Codes 23 Freedonia Methodology 23 Resources 25 Rigid Intermediate Bulk Containers: United States 1 ©2019 The Freedonia Group. All rights reserved. List of Tables & Figures Figure 1 | Key Trends in the US Rigid Intermediate Bulk Container Market, 2018 – 2023 3 Figure 2 | US Rigid Intermediate Bulk Container Demand Trends, 2008 – 2018 4 Table 1 | Key Indicators for US Rigid Intermediate Bulk Container Demand, 2008 – 2023 (US$ bil) 5 Figure 3 | US Rigid Intermediate Bulk Container Demand by Product, 2008 – 2023 (US$ mil) 10 Table 2 | US Rigid Intermediate Bulk Container Demand by Product, 2008 – 2023 (US$ mil) 10 Figure 4 | US Rigid Intermediate Bulk Container Demand by Product, 2008 – 2023 (%) 12 Figure 5 | US Rigid Intermediate Bulk Container Demand by Market, 2008 – 2023 (US$ mil) 14 Table 3 | US Rigid Intermediate Bulk Container Demand by Market, 2008 – 2023 (US$ mil) 14 Figure 6 | US Rigid Intermediate Bulk Container Demand by Market, 2008 – 2023 (%) 18 Table 4 | Leading Suppliers in the US Rigid Intermediate Bulk Container Market by Product 20 Table 5 | NAICS & SIC Codes Related to Rigid Intermediate Bulk Containers 23 Rigid Intermediate Bulk Containers: United States 2 ©2019 The Freedonia Group. -

LOWERING COSTS and SIMPLIFYING WORKFLOW with the Automate 2550 SYSTEM
HACETTEPE UNIVERSITY FACULTY OF MEDICINE HOSPITAL CENTRAL AND STAT LABORATORIES LOWERING COSTS AND SIMPLIFYING WORKFLOW WITH THE AutoMate 2550 SYSTEM Laboratory profile › 1,040-bed hospital located in Ankara, Turkey › Provides uninterrupted 24/7 inpatient, outpatient and emergency services › Employs 6 professors, 6 associate Hacettepe University Faculty of Medicine Hospital is one of the leading professors and 130 operators university hospitals in Turkey. The university is well known for its › Performs up to 6.5 million geographical location and adoption of ground breaking technologies. biochemistry and hormone While the reference site is preferred by a large number of patients, it is tests annually of particular interest to specific patient populations seeking test results › Processes up to 2 million tests from a variety of panels. With many different systems performing on instruments in more than hundreds of standard and specialized tests, the hospital’s laboratories 20 different units are driven to ensure the delivery of results without error, while › Operates a Beckman Coulter containing costs and managing workload burden. standalone AutoMate 2550 pre- For more than 16 years, the facility—which has been held up as an and post-analytical automation system in the biochemistry example of excellence in its own region, as well as all over the country— laboratory, along with a Power has been using a standalone automation system with an aliquoter Processor system with two feature. With this technology, it has enhanced patient satisfaction, -
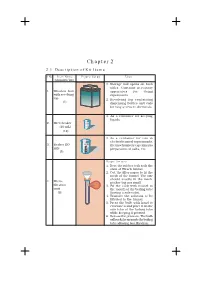
Chapter 2 2.1 Description of Kit Items
Manual of SEAT Friendly MCL Kit 7 Chapter 2 2.1 Description of Kit Items S.No. Item Name Figure/Setup Uses (Quantity/ kit) 1. Storage box opens on both sides. Contains necessary 1. Wooden box apparatus for doing with revolving experiments. top 2. Revolving top containing (1) dispensing bottles and vials for easy access to chemicals. 1. As a container for keeping liquids. 2. Microbeaker (10 mL) (12) 1. As a container for use in electrochemical experiments, 3. Beaker (50 thermochemistry experiments mL) preparation of salts, etc. (3) Steps for use 1. Bore the rubber cork to fit the stem of Hirsch funnel. 2. Cut the filter paper to fit the mesh of the funnel. The size should exactly fit the mesh, 4. Micro- neither big nor small. filtration 3. Fit the cork with funnel in unit the mouth of the boiling tube (2) (having a side tube). 4. Transfer the solution to be filtrated to the funnel. 5. Press the bulb with hand to evacuate it and place it on the side tube of the boiling tube while keeping it pressed. 6. Release the pressure. The bulb will suck the air inside the boiling tube allowing fast filtration. 8 Manual of SEAT Friendly MCL Kit S.No. Item Name Figure/Setup Uses (Quantity/Kit) 1. The watch glass is used to hold solids when being 5. Watch glass weighed. (2) 2. It should never be heated. 1. Flat plate with multiple “wells” used as micro test tubes. 2. Contains 96 rectangular matrix. 3. Each well of a micro plate typically holds somewhere between a few to a few hundred microlitres of liquid. -

Laboratory Supplies and Equipment
Laboratory Supplies and Equipment Beakers: 9 - 12 • Beakers with Handles • Printed Square Ratio Beakers • Griffin Style Molded Beakers • Tapered PP, PMP & PTFE Beakers • Heatable PTFE Beakers Bottles: 17 - 32 • Plastic Laboratory Bottles • Rectangular & Square Bottles Heatable PTFE Beakers Page 12 • Tamper Evident Plastic Bottles • Concertina Collapsible Bottle • Plastic Dispensing Bottles NEW Straight-Side Containers • Plastic Wash Bottles PETE with White PP Closures • PTFE Bottle Pourers Page 39 Containers: 38 - 42 • Screw Cap Plastic Jars & Containers • Snap Cap Plastic Jars & Containers • Hinged Lid Plastic Containers • Dispensing Plastic Containers • Graduated Plastic Containers • Disposable Plastic Containers Cylinders: 45 - 48 • Clear Plastic Cylinder, PMP • Translucent Plastic Cylinder, PP • Short Form Plastic Cylinder, PP • Four Liter Plastic Cylinder, PP NEW Polycarbonate Graduated Bottles with PP Closures Page 21 • Certified Plastic Cylinder, PMP • Hydrometer Jar, PP • Conical Shape Plastic Cylinder, PP Disposal Boxes: 54 - 55 • Bio-bin Waste Disposal Containers • Glass Disposal Boxes • Burn-upTM Bins • Plastic Recycling Boxes • Non-Hazardous Disposal Boxes Printed Cylinders Page 47 Drying Racks: 55 - 56 • Kartell Plastic Drying Rack, High Impact PS • Dynalon Mega-Peg Plastic Drying Rack • Azlon Epoxy Coated Drying Rack • Plastic Draining Baskets • Custom Size Drying Racks Available Burn-upTM Bins Page 54 Dynalon® Labware Table of Contents and Introduction ® Dynalon Labware, a leading wholesaler of plastic lab supplies throughout