BMJ Open Is Committed to Open Peer Review. As Part of This Commitment We Make the Peer Review History of Every Article We Publish Publicly Available
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CDR Clinical Review Report for Soliqua
CADTH COMMON DRUG REVIEW Clinical Review Report Insulin glargine and lixisenatide injection (Soliqua) (Sanofi-Aventis) Indication: adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus inadequately controlled on basal insulin (less than 60 units daily) alone or in combination with metformin. Service Line: CADTH Common Drug Review Version: Final (with redactions) Publication Date: January 2019 Report Length: 118 Pages Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services. While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. -

Download Product Insert (PDF)
PRODUCT INFORMATION Remogliflozin A Item No. 14340 CAS Registry No.: 329045-45-6 OH Formal Name: 5-methyl-4-[[4-(1-methylethoxy) N O OH phenyl]methyl]-1-(1-methylethyl)- N 1H-pyrazol-3-yl β-D- O glucopyranoside OH Synonym: GSK189074 OH MF: C23H34N2O7 FW: 450.5 Purity: ≥98% λ: 229 nm UV/Vis.: max O Supplied as: A crystalline solid Storage: -20°C Stability: ≥2 years Information represents the product specifications. Batch specific analytical results are provided on each certificate of analysis. Laboratory Procedures Remogliflozin A is supplied as a crystalline solid. A stock solution may be made by dissolving the remogliflozin A in the solvent of choice. Remogliflozin A is soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide, which should be purged with an inert gas. The solubility of remogliflozin A in these solvents is approximately 30 mg/ml. Remogliflozin A is sparingly soluble in aqueous buffers. For maximum solubility in aqueous buffers, remogliflozin A should first be dissolved in ethanol and then diluted with the aqueous buffer of choice. Remogliflozin A has a solubility of approximately 0.5 mg/ml in a 1:1 solution of ethanol:PBS (pH 7.2) using this method. We do not recommend storing the aqueous solution for more than one day. Description Remogliflozin A is a potent inhibitor of sodium-glucose transporter 2 (SGLT2; Kis = 12.4 and 26 nM 1 for human and rat SGLT2, respectively). It is selective for SGLT2 over SGLT1 (Kis = 4,520 and 997 nM for human and rat SGLT1, respectively). Following administration of a prodrug, remogliflozin etabonate, that is rapidly converted to remogliflozin A in vivo, rat urinary glucose excretion increases and plasma glucose and insulin concentrations decrease. -

Remogliflozin Etabonate, a Selective Inhibitor of the Sodium-Glucose
Clinical Care/Education/Nutrition/Psychosocial Research BRIEF REPORT Remogliflozin Etabonate, a Selective Inhibitor of the Sodium-Glucose Transporter 2, Improves Serum Glucose Profiles in Type 1 Diabetes 1,2 3 SUNDER MUDALIAR, MD JUNE YE, PHD placebo (placebo), 2) mealtime insulin 1 3 DEBRA A. ARMSTRONG, BA, RN, CCRC ELIZABETH K. HUSSEY, PHARMD 2 3 injection + RE placebo (prandial insulin), ANNIE A. MAVIAN, MD DEREK J. NUNEZ, MD 3 3 1,2 ) placebo insulin injection + 50 mg RE (RE ROBIN O’CONNOR-SEMMES, PHD ROBERT R. HENRY, MD 3 3 50 mg), 4) placebo insulin injection + 150 PATRICIA K. MYDLOW, BS ROBERT L. DOBBINS, MD, PHD mg RE (RE 150 mg), and 5) placebo insulin injection + 500 mg RE (RE 500 mg). d fl Each individual received 75-g oral OBJECTIVES Remogli ozin etabonate (RE), an inhibitor of the sodium-glucose transporter glucose and identical meals during all 2, improves glucose profiles in type 2 diabetes. This study assessed safety, tolerability, pharma- cokinetics, and pharmacodynamics of RE in subjects with type 1 diabetes. treatment periods. Frequent samples were obtained for measurement of plasma RESEARCH DESIGN AND METHODSdTen subjects managed with continuous sub- glucose and insulin concentrations. Urine cutaneous insulin infusion were enrolled. In addition to basal insulin, subjects received five samples were collected for 24 h to assess randomized treatments: placebo, prandial insulin, 50 mg RE, 150 mg RE, and mg RE 500. creatinine clearance and glucose excre- d tion. Plasma samples were collected for RESULTS Adverse events and incidence of hypoglycemia with RE did not differ from placebo fl and prandial insulin groups. -
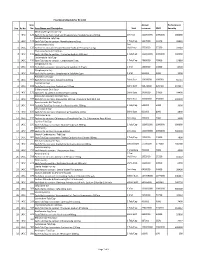
Sno. Rc No Item No. Item Name and Description Unit Annual Turnover
Final Drug Schedule for RC 145C Item Annual Performance Sno. Rc No No. Item Name and Description Unit turnover EMD Security Medroxy Progesterone Inj- 1 145C 126 Each ml to contain: Medroxy Progesterone Acetate Suspn.150mg. 1ml Vial 100000000 1000000 600000 Norethisterone Tab/Cap- 2 145C 128 Each Tab/Cap to contain: Norethisterone 5mg. 1 Tab/Cap 5637000 56370 33822 Dexamethasone Inj- 2ml 3 145C 133 Each ml to contain: Dexamethasone Sodium Phosphate 4 mg. Vial/Amp 5735000 57350 34410 Thyroxine Sodium Tab/Cap- 4 145C 135 Each Tab/Cap to contain: Thyroxine Sodium 100 mcg 1 Tab/Cap 100000000 1000000 600000 Carbimazole Tab/Cap- 5 145C 136 Each Tab/Cap to contain: Carbimazole 5 mg. 1 Tab/Cap 2980000 29800 17880 Streptomycin Inj- 6 145C 151 Each Vial to contain: Streptomycin Sulphate 0.75gm 1 Vial 1400000 14000 8400 Streptomycin Inj- 7 145C 152 Each Vial to contain: Streptomycin Sulphate 1gm 1 Vial 500000 5000 3000 Ampicillin Dry Syp- 8 145C 161 Each 5ml to contain: Ampicillin 125mg 30ml Bott 10959000 109590 65754 Cephalexin Syp- 9 145C 168 Each 5ml to contain: Cephalexin 125mg 30ml Bott 32874000 328740 197244 Erthyromycin Oral Susp- 10 145C 172 Each 5ml to contain: Erythromycin 125mg 30ml Bott 2400000 24000 14400 Amoxy & Clavulanic Acid Dry Syp- 11 145C 183 Each 5ml to contain: Amoxycillin 200 mg, Clavulanic Acid 28.5 mg 30ml Bott 35000000 350000 210000 Pyrazinamide Kid Tab/Cap- 12 145C 191 Each Kid Tab/Cap to contain: Pyrazinamide 300mg 1 Tab/Cap 500000 5000 3000 Chloroquine Syp- 13 145C 196 Each 5ml to contain: Chloroquine Phosphate 50mg. -

Exploring Glycosuria As a Mechanism for Weight and Fat Mass Reduction. a Pilot Study with Remogliflozin Etabonate and Sergliflozin Etabonate in Healthy Obese Subjects
Journal of Clinical & Translational Endocrinology 1 (2014) e3ee8 Contents lists available at ScienceDirect Journal of Clinical & Translational Endocrinology journal homepage: www.elsevier.com/locate/jcte Research Paper Exploring glycosuria as a mechanism for weight and fat mass reduction. A pilot study with remogliflozin etabonate and sergliflozin etabonate in healthy obese subjects Antonella Napolitano a,*, Sam Miller a, Peter R. Murgatroyd c, Elizabeth Hussey b, Robert L. Dobbins b, Edward T. Bullmore a, Derek J.R. Nunez b View metadata, citation and similar papers at core.ac.uk brought to you by CORE a Clinical Unit in Cambridge, GlaxoSmithKline, Addenbrookes Hospital, Cambridge, UK b Metabolic Pathways and Cardiovascular Unit, GlaxoSmithKline,provided by NC, Elsevier USA - Publisher Connector c Wellcome Trust Clinical Research Facility, Cambridge University Hospital NHS Trust, UK article info abstract Article history: Inhibitors of sodium-dependent glucose co-transporter 2 (SGLT2) increase glucose excretion in the urine Received 16 October 2013 and improve blood glucose in Type 2 diabetes mellitus. Glycosuria provides an energy and osmotic drain Received in revised form that could alter body composition. We therefore conducted a pilot study comparing the effects on body 26 November 2013 composition of two SGLT2 inhibitors, remogliflozin etabonate (RE) 250 mg TID (n ¼ 9) and sergliflozin Accepted 5 December 2013 etabonate (SE) (1000 mg TID) (n ¼ 9), with placebo (n ¼ 12) in obese non-diabetic subjects. Both drugs Available online 7 February 2014 were well tolerated during 8 weeks of dosing, and the most common adverse event was headache. No urinary tract infections were observed, but there was one case of vaginal candidiasis in the RE group. -

(SGLT2) Inhibitors: a Systematic Review and Meta-Analysis
Open access Research BMJ Open: first published as 10.1136/bmjopen-2018-022577 on 1 February 2019. Downloaded from Comparative safety of the sodium glucose co-transporter 2 (SGLT2) inhibitors: a systematic review and meta-analysis Jennifer R Donnan,1 Catherine A Grandy,1 Eugene Chibrikov,1 Carlo A Marra,1,2 Kris Aubrey-Bassler,3 Karissa Johnston,1 Michelle Swab,3 Jenna Hache,1 Daniel Curnew,1 Hai Nguyen,1 John-Michael Gamble1,4 To cite: Donnan JR, Grandy CA, ABSTRACT Strengths and limitations of this study Chibrikov E, et al. Comparative Objective To estimate the association between the use safety of the sodium glucose of sodium glucose co-transporter-2 (SGLT2) inhibitors ► This study provides a comprehensive systematic co-transporter 2 (SGLT2) and postmarket harms as identified by drug regulatory inhibitors: a systematic review review of potential serious adverse events related agencies. and meta-analysis. BMJ Open to use of sodium glucose co-transporter-2 (SGLT2) Design We conducted a systematic review and meta- 2019;9:e022577. doi:10.1136/ inhibitors identified by drug regulatory agencies. analysis of randomised controlled trials (RCT). Six large bmjopen-2018-022577 ► This study considered select outcomes to provide databases were searched from inception to May 2018. focused attention on the issues concerning regula- ► Prepublication history and Random effects models were used to estimate pooled tors; however, this means that additional knowledge additional material for this relative risks (RRs). paper are available online. To of the clinical benefits and harms needs to be con- Intervention SGLT2 inhibitors, compared with placebo or view these files, please visit sidered before applying the results of this study. -

Efficacy and Safety of Remogliflozin Etabonate, a New Sodium Glucose
Drugs (2020) 80:587–600 https://doi.org/10.1007/s40265-020-01285-0 ORIGINAL RESEARCH ARTICLE Efcacy and Safety of Remoglifozin Etabonate, a New Sodium Glucose Co‑Transporter‑2 Inhibitor, in Patients with Type 2 Diabetes Mellitus: A 24‑Week, Randomized, Double‑Blind, Active‑Controlled Trial Mala Dharmalingam1 · S. R. Aravind2 · Hemant Thacker3 · S. Paramesh4 · Brij Mohan5 · Manoj Chawla6 · Arthur Asirvatham7 · Ramesh Goyal8 · Jayashri Shembalkar9 · R. Balamurugan10 · Pradnya Kadam11 · Hansraj Alva12 · Rahul Kodgule13 · Monika Tandon13 · Sivakumar Vaidyanathan13 · Amol Pendse13 · Rajesh Gaikwad13 · Sagar Katare13 · Sachin Suryawanshi13 · Hanmant Barkate13 Published online: 11 March 2020 © The Author(s) 2020 Abstract Background Metformin is the frst-line treatment for type 2 diabetes mellitus (T2DM), but many patients either cannot tolerate it or cannot achieve glycemic control with metformin alone, so treatment with other glucose-lowering agents in combination with metformin is frequently required. Remoglifozin etabonate, a novel agent, is an orally bioavailable prodrug of remoglifozin, which is a potent and selective sodium-glucose co-transporter-2 inhibitor. Objective Our objective was to evaluate the efcacy and safety of remoglifozin etabonate compared with dapaglifozin in subjects with T2DM in whom a stable dose of metformin as monotherapy was providing inadequate glycemic control. Methods A 24-week randomized, double-blind, double-dummy, active-controlled, three-arm, parallel-group, multi- center, phase III study was conducted in India. Patients aged ≥ 18 and ≤ 65 years diagnosed with T2DM, receiving met- formin ≥ 1500 mg/day, and with glycated hemoglobin (HbA1c) levels ≥ 7 to ≤ 10% at screening were randomized into three groups. Every patient received metformin ≥ 1500 mg and either remoglifozin etabonate 100 mg twice daily (BID) (group 1, n = 225) or remoglifozin etabonate 250 mg BID (group 2, n = 241) or dapaglifozin 10 mg once daily (QD) in the morning and placebo QD in the evening (group 3, n = 146). -

Canagliflozin, Dapagliflozin and Empagliflozin Monotherapy for Treating Type 2 Diabetes: Systematic Review and Economic Evaluation
HEALTH TECHNOLOGY ASSESSMENT VOLUME 21 ISSUE 2 JANUARY 2017 ISSN 1366-5278 Canagliflozin, dapagliflozin and empagliflozin monotherapy for treating type 2 diabetes: systematic review and economic evaluation Rhona Johnston, Olalekan Uthman, Ewen Cummins, Christine Clar, Pamela Royle, Jill Colquitt, Bee Kang Tan, Andrew Clegg, Saran Shantikumar, Rachel Court, J Paul O’Hare, David McGrane, Tim Holt and Norman Waugh DOI 10.3310/hta21020 Canagliflozin, dapagliflozin and empagliflozin monotherapy for treating type 2 diabetes: systematic review and economic evaluation Rhona Johnston,1 Olalekan Uthman,2 Ewen Cummins,1 Christine Clar,3 Pamela Royle,2 Jill Colquitt,4 Bee Kang Tan,2 Andrew Clegg,5 Saran Shantikumar,2 Rachel Court,2 J Paul O’Hare,2 David McGrane,6 Tim Holt7 and Norman Waugh2* 1McMDC, Harrogate, UK 2Warwick Evidence, Division of Health Sciences, University of Warwick, Coventry, UK 3Berlin, Germany 4Effective Evidence, Waterlooville, UK 5University of Central Lancashire, Preston, UK 6Queen Elizabeth University Hospital, Glasgow, UK 7University of Oxford, Oxford, UK *Corresponding author Declared competing interests of authors: David McGrane has spoken at educational meetings sponsored by AstraZeneca, Eli Lilly, Sanofi, MSD, Takeda Pharmaceutical Company, Novo Nordisk, Janssen, and has served on Advisory Boards for Eli Lilly, Sanofi, Novo Nordisk. J Paul O’Hare has received lecture fees, advisory board meeting fees, and grants for research from Novo Nordisk and Sanofi. All fees are paid through University of Warwick to fund access to insulin projects in sub-Saharan Africa. Published January 2017 DOI: 10.3310/hta21020 This report should be referenced as follows: Johnston R, Uthman O, Cummins E, Clar C, Royle P, Colquitt J, et al. -

Glenmark Receives Approval for Combination of Remogliflozin Etabonate and Metformin Hydrochloride for Adults with Type 2 Diabetes in India
Press Release For Immediate Release Glenmark receives approval for combination of Remogliflozin Etabonate and Metformin Hydrochloride for adults with type 2 diabetes in India Glenmark will commercialize the combination under the brand names ‘Remo-M’ and ‘Remozen-M’ Earlier in April 2019, Glenmark received regulatory approval for Remogliflozin etabonate in India and became the first company in the world to launch Remogliflozin with India being the first country to get access to this innovative drug Remogliflozin is an innovative, patent-protected sodium glucose co-transporter-2 (SGLT2) inhibitor indicated in treatment of type-2 diabetes mellitus in adults Mumbai, India; August 19, 2019: Glenmark Pharmaceuticals Ltd. (Glenmark), a research-led global integrated pharmaceutical company, today announced that the company has received regulatory approval to market a combination of its novel, patent protected and globally-researched sodium glucose co-transporter-2 (SGLT2) inhibitor Remogliflozin etabonate (Remogliflozin) and Metformin Hydrochloride (Metformin) film coated tablets in India. The drug is indicated in the treatment of type-2 diabetes mellitus in adults. Glenmark will commercialize the product under the brand names ‘Remo-M’ and Remozen-M’. Earlier in April 2019, Glenmark received regulatory approval for Remogliflozin etabonate 100 mg tablets, twice daily, after successfully completing Phase-3 clinical trials in which Remogliflozin demonstrated good efficacy and safety profile in a head-to-head comparison against Dapagliflozin. With this approval Glenmark became the first company in the world to launch the novel SGLT2 inhibitor Remogliflozin with India being the first country to get access to this innovative drug. Glenmark subsequently launched Remogliflozin in India under the brand names ‘Remo’ and ‘Remozen’. -

Download Product Insert (PDF)
PRODUCT INFORMATION Remogliflozin etabonate Item No. 14341 CAS Registry No.: 442201-24-3 OH Formal Name: 5-methyl-4-[[4-(1-methylethoxy)phenyl] N O OH methyl]-1-(1-methylethyl)-1H-pyrazol-3-yl, N O β-D-glucopyranoside 6-(ethyl carbonate) OH Synonym: GSK189075 MF: C26H38N2O9 O FW: 522.6 O Purity: ≥98% O Supplied as: A solid O Storage: -20°C Stability: ≥2 years Information represents the product specifications. Batch specific analytical results are provided on each certificate of analysis. Laboratory Procedures Remogliflozin etabonate is supplied as a solid. A stock solution may be made by dissolving the remogliflozin etabonate in the solvent of choice, which should be purged with an inert gas. Remogliflozin etabonate is soluble in methanol and DMSO. Description Remogliflozin etabonate is a prodrug form of the sodium-glucose transporter 2 (SGLT2) inhibitor remogliflozin A (Item No. 14340).1 Remogliflozin etabonate inhibits human SGLT2 and SGLT1 (Kis = 1.95 and 43.1 μM, respectively). It inhibits increases in plasma glucose levels in a glucose tolerance test in a rat model of diabetes induced by streptozotocin (STZ; Item No. 13104) when administered at doses of 3 and 10 mg/ml. Remogliflozin etabonate (10 and 30 mg/kg twice per day for 6 weeks) also increases fasting plasma insulin levels and reduces fasting plasma glucose and triglyceride levels, as well as urinary glucose excretion, in a db/db mouse model of diabetes with hyperinsulinemia and obesity. Reference 1. Fujimori, Y., Katsuno, K., Nakashima, I., et al. Remogliflozin etabonate, in a novel category of selective low-affinity sodium glucose cotransporter (SGLT2) inhibitors, exhibits antidiabetic efficacy in rodent models. -

Adverse Effects and Safety of SGLT2 Inhibitor Use Among Patients with Type 2 Diabetes: Findings from RCT Evidence
78 Apr 2017 Vol 10 No.2 North American Journal of Medicine and Science Review Adverse Effects and Safety of SGLT2 Inhibitor Use among Patients with Type 2 Diabetes: Findings from RCT Evidence Huilin Tang, MSc;1,2,3 Jingjing Zhang, MD;4 Yiqing Song, MD, ScD2,3* 1 Department of Pharmacy, Peking University Third Hospital, Beijing, China 2 Department of Epidemiology, Richard M. Fairbanks School of Public Health, Indiana University, Indianapolis, IN 3 Center for Pharmacoepidemiology, Richard M. Fairbanks School of Public Health, Indiana University, Indianapolis, IN 4 Division of Nephrology, Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, PA Sodium-glucose cotransporter 2 (SGTL2) inhibitors, a novel class of glucose-lowering agents, act in an insulin-independent manner by increasing urinary glucose excretion. In addition to reduce hyperglycemia, SGTL2 inhibitor exerts beneficial effects on cardiovascular risk factors (e.g., lower blood pressure and enhance weigh loss), which may confer additional health benefits for type 2 diabetes patients. The EMPA- REG OUTCOME trial showed that empagliflozin not only reduced the risk of major adverse cardiovascular events but also slowed down the progression of kidney disease compared with placebo. However, some evidence indicated an increased risk of composite renal events among patients using dapagliflozin. The beneficial cardiovascular and renal effects of EMPA-REG OUTCOME trial representing a class effect or a specific drug effect warrants to be further investigated. SGLT2 inhibitors were associated with increased risks of genital mycotic infections and urinary tract infections. Some mechanisms indicated that SGLT2 inhibitor might lead to diabetic ketoacidosis and bone fracture. These risks remain uncertain, though some evidence from the meta-analyses did not find any significantly increased risks of diabetic ketoacidosis and bone fracture. -

Reniva Monograph
Product Monograph Table of Contents PART I: HEALTH PROFESSIONAL INFORMATION 1 SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT 1 2. QUALITATIVE AND QUANTITATIVE COMPOSITION 1 3. PHARMACEUTICAL FORM 1 4. CLINICAL PARTICULARS 1 4.1 Therapeutic indications 1 4.2 Posology and Method of Administration 1 4.3 Contraindications 2 4.4 Special Warnings and Precautions for Use 2 4.5 Interaction with other medicinal products and other forms of interactions 3 4.6 Fertility, pregnancy and lactation 4 4.7 Effects on ability to drive and use machines 5 4.8 Undesirable effects 5 4.9 Overdose 8 5. PHARMACOLOGICAL PROPERTIES 8 5.1 Pharmacodynamic properties 8 5.2 Pharmacokinetic properties 10 6. PHARMACEUTICAL PARTICULARS 11 6.1 List of excipients 11 6.2 Incompatibilities 11 6.3 Shelf Life 11 6.4 Special Precautions for Storage 11 6.5 Nature and Contents of the Container 11 PART II: SCIENTIFIC INFORMATION 12 1. PHARMACEUTICAL INFORMATION 12 2. CLINICAL TRIALS 12 2.1. Clinical Studies and designs 13 2.2. Study Results 16 3. DETAILED PHARMACOLOGY 20 4. TOXICOLOGY 34 5. REFERENCES 38 PART I: HEALTH PROFESSIONAL INFORMATION SUMMARY OF PRODUCT CHARACTERISTICS Remogliflozin etabonate 100 mg Tablets 1. NAME OF THE MEDICINAL PRODUCT Remogliflozin etabonate 100 mg Tablets 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Remogliflozin etabonate 100 mg Tablets Each film coated tablet contains: Remogliflozin Etabonate …. 100 mg Excipients q.s Colour: Titanium Dioxide USP. 3. PHARMACEUTICAL FORM Film coated tablets 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Remogliflozin etabonate is indicated in adults aged 18 years and older with type 2 diabetes mellitus to improve glycemic control as: • Monotherapy when diet and exercise alone do not provide adequate glycaemic control.