(SGLT2) Inhibitors: a Systematic Review and Meta-Analysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Summary of Investigation Results Sodium-Glucose Co-Transporter 2 (SGLT2) Inhibitors
Pharmaceuticals and Medical Devices Agency This English version is intended to be a reference material for the convenience of users. In the event of inconsistency between the Japanese original and this English translation, the former shall prevail. Summary of investigation results Sodium-glucose co-transporter 2 (SGLT2) inhibitors September 15, 2015 Non-proprietary name a. Canagliflozin hydrate b. Dapagliflozin propylene glycolate hydrate c. Empagliflozin d. Ipragliflozin L-proline e. Luseogliflozin hydrate f. Tofogliflozin hydrate Brand name (Marketing authorization holder) a. Canaglu Tablets 100 mg (Mitsubishi Tanabe Pharma Corporation) b. Forxiga Tablets 5 mg and 10 mg (AstraZeneca K.K.) c. Jardiance Tablets 10 mg and 25 mg (Nippon Boehringer Ingelheim Co., Ltd.) d. Suglat Tablets 25 mg and 50 mg (Astellas Pharma Inc.) e. Lusefi Tablets 2.5 mg and 5 mg (Taisho Pharmaceutical Co., Ltd.) f. Apleway Tablets 20 mg (Sanofi K.K.) and Deberza Tablets 20 mg (Kowa Company, Ltd.) Indications Type 2 diabetes mellitus Summary of revision 1. Precautions regarding ketoacidosis should be added in the Important Precautions section for the above products from a to f. 2. “Ketoacidosis” should be newly added in the Clinically significant adverse reaction section for the above products from a to f. 3. “Sepsis” should be added to the “Pyelonephritis” subsection in the Important Precautions section for the above products from a to f. Pharmaceuticals and Medical Devices Agency Office of Safety I 3-3-2 Kasumigaseki, Chiyoda-ku, Tokyo 100-0013 Japan E-mail: [email protected] Pharmaceuticals and Medical Devices Agency This English version is intended to be a reference material for the convenience of users. -

Modifications to the Harmonized Tariff Schedule of the United States to Implement Changes to the Pharmaceutical Appendix
United States International Trade Commission Modifications to the Harmonized Tariff Schedule of the United States to Implement Changes to the Pharmaceutical Appendix USITC Publication 4208 December 2010 U.S. International Trade Commission COMMISSIONERS Deanna Tanner Okun, Chairman Irving A. Williamson, Vice Chairman Charlotte R. Lane Daniel R. Pearson Shara L. Aranoff Dean A. Pinkert Address all communications to Secretary to the Commission United States International Trade Commission Washington, DC 20436 U.S. International Trade Commission Washington, DC 20436 www.usitc.gov Modifications to the Harmonized Tariff Schedule of the United States to Implement Changes to the Pharmaceutical Appendix Publication 4208 December 2010 (This page is intentionally blank) Pursuant to the letter of request from the United States Trade Representative of December 15, 2010, set forth at the end of this publication, and pursuant to section 1207(a) of the Omnibus Trade and Competitiveness Act, the United States International Trade Commission is publishing the following modifications to the Harmonized Tariff Schedule of the United States (HTS) to implement changes to the Pharmaceutical Appendix, effective on January 1, 2011. Table 1 International Nonproprietary Name (INN) products proposed for addition to the Pharmaceutical Appendix to the Harmonized Tariff Schedule INN CAS Number Abagovomab 792921-10-9 Aclidinium Bromide 320345-99-1 Aderbasib 791828-58-5 Adipiplon 840486-93-3 Adoprazine 222551-17-9 Afimoxifene 68392-35-8 Aflibercept 862111-32-8 Agatolimod -

CDR Clinical Review Report for Soliqua
CADTH COMMON DRUG REVIEW Clinical Review Report Insulin glargine and lixisenatide injection (Soliqua) (Sanofi-Aventis) Indication: adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus inadequately controlled on basal insulin (less than 60 units daily) alone or in combination with metformin. Service Line: CADTH Common Drug Review Version: Final (with redactions) Publication Date: January 2019 Report Length: 118 Pages Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services. While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. -

Download Product Insert (PDF)
PRODUCT INFORMATION Remogliflozin A Item No. 14340 CAS Registry No.: 329045-45-6 OH Formal Name: 5-methyl-4-[[4-(1-methylethoxy) N O OH phenyl]methyl]-1-(1-methylethyl)- N 1H-pyrazol-3-yl β-D- O glucopyranoside OH Synonym: GSK189074 OH MF: C23H34N2O7 FW: 450.5 Purity: ≥98% λ: 229 nm UV/Vis.: max O Supplied as: A crystalline solid Storage: -20°C Stability: ≥2 years Information represents the product specifications. Batch specific analytical results are provided on each certificate of analysis. Laboratory Procedures Remogliflozin A is supplied as a crystalline solid. A stock solution may be made by dissolving the remogliflozin A in the solvent of choice. Remogliflozin A is soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide, which should be purged with an inert gas. The solubility of remogliflozin A in these solvents is approximately 30 mg/ml. Remogliflozin A is sparingly soluble in aqueous buffers. For maximum solubility in aqueous buffers, remogliflozin A should first be dissolved in ethanol and then diluted with the aqueous buffer of choice. Remogliflozin A has a solubility of approximately 0.5 mg/ml in a 1:1 solution of ethanol:PBS (pH 7.2) using this method. We do not recommend storing the aqueous solution for more than one day. Description Remogliflozin A is a potent inhibitor of sodium-glucose transporter 2 (SGLT2; Kis = 12.4 and 26 nM 1 for human and rat SGLT2, respectively). It is selective for SGLT2 over SGLT1 (Kis = 4,520 and 997 nM for human and rat SGLT1, respectively). Following administration of a prodrug, remogliflozin etabonate, that is rapidly converted to remogliflozin A in vivo, rat urinary glucose excretion increases and plasma glucose and insulin concentrations decrease. -

Effect of Luseogliflozin on Bone Microarchitecture in Elderly Patients with Type 2 Diabetes
Effect of luseogliozin on bone microarchitecture in elderly patients with type 2 diabetes: Study protocol for a randomized controlled trial using second- generation high-resolution peripheral quantitative computed tomography (HR-pQCT) Ai Haraguchi Nagasaki Daigaku https://orcid.org/0000-0002-0671-2530 Riyoko Shigeno Nagasaki Daigaku Ichiro Horie ( [email protected] ) https://orcid.org/0000-0003-3430-5796 Shimpei Morimoto Nagasaki Daigaku Ayako Ito Nagasaki Daigaku Ko Chiba Nagasaki Daigaku Yurika Kawazoe Nagasaki Daigaku Shigeki Tashiro Nagasaki Daigaku Junya Miyamoto Nagasaki Daigaku Shuntaro Sato Nagasaki Daigaku Hiroshi Yamamoto Nagasaki Daigaku Makoto Osaki Nagasaki Daigaku Atsushi Kawakami Nagasaki Daigaku Norio Abiru Nagasaki Daigaku Page 1/18 Study protocol Keywords: type 2 diabetes, luseogliozin, SGLT2 inhibitor, HR-pQCT, fracture, bone Posted Date: September 6th, 2019 DOI: https://doi.org/10.21203/rs.2.14017/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published at Trials on May 5th, 2020. See the published version at https://doi.org/10.1186/s13063-020-04276-4. Page 2/18 Abstract Background Elderly patients with type 2 diabetes mellitus (T2DM) have an increased risk of bone fracture independent of their bone mineral density (BMD), which is explained mainly by the deteriorated bone quality in T2DM compared to non-diabetic adults. Sodium-glucose co-transporter (SGLT) 2 inhibitors have been studied in several trials in T2DM, and the Canagliozin Cardiovascular Assessment Study showed an increased fracture risk related to treatment with the SGLT2 inhibitor canagliozin, although no evidence of increased fracture risk with treatment with other SGLT2 inhibitors has been reported. -

Remogliflozin Etabonate, a Selective Inhibitor of the Sodium-Glucose
Clinical Care/Education/Nutrition/Psychosocial Research BRIEF REPORT Remogliflozin Etabonate, a Selective Inhibitor of the Sodium-Glucose Transporter 2, Improves Serum Glucose Profiles in Type 1 Diabetes 1,2 3 SUNDER MUDALIAR, MD JUNE YE, PHD placebo (placebo), 2) mealtime insulin 1 3 DEBRA A. ARMSTRONG, BA, RN, CCRC ELIZABETH K. HUSSEY, PHARMD 2 3 injection + RE placebo (prandial insulin), ANNIE A. MAVIAN, MD DEREK J. NUNEZ, MD 3 3 1,2 ) placebo insulin injection + 50 mg RE (RE ROBIN O’CONNOR-SEMMES, PHD ROBERT R. HENRY, MD 3 3 50 mg), 4) placebo insulin injection + 150 PATRICIA K. MYDLOW, BS ROBERT L. DOBBINS, MD, PHD mg RE (RE 150 mg), and 5) placebo insulin injection + 500 mg RE (RE 500 mg). d fl Each individual received 75-g oral OBJECTIVES Remogli ozin etabonate (RE), an inhibitor of the sodium-glucose transporter glucose and identical meals during all 2, improves glucose profiles in type 2 diabetes. This study assessed safety, tolerability, pharma- cokinetics, and pharmacodynamics of RE in subjects with type 1 diabetes. treatment periods. Frequent samples were obtained for measurement of plasma RESEARCH DESIGN AND METHODSdTen subjects managed with continuous sub- glucose and insulin concentrations. Urine cutaneous insulin infusion were enrolled. In addition to basal insulin, subjects received five samples were collected for 24 h to assess randomized treatments: placebo, prandial insulin, 50 mg RE, 150 mg RE, and mg RE 500. creatinine clearance and glucose excre- d tion. Plasma samples were collected for RESULTS Adverse events and incidence of hypoglycemia with RE did not differ from placebo fl and prandial insulin groups. -

For the Use of a Registered Medical Practitioner Or a Hospital Or a Laboratory Only TENELIGLIPTIN, PIOGLITAZONE HYDROCHLORIDE AN
For the use of a Registered Medical Practitioner or a Hospital or a Laboratory only TENELIGLIPTIN, PIOGLITAZONE HYDROCHLORIDE AND PROLONGED RELEASE METFORMIN HYDROCHLORIDE TABLETS 1. THE DRUG SHOULD BE USED AT FIRST LINE OF THERAPY FOR DIABETES. 2. ADVICE FOR HEALTHCARE PROFESSIONALS • Patients with active bladder cancer or with a history of bladder cancer and those with uninvestigated haematuria should not receive pioglitazone. • Prescribers should review the safety and efficacy of pioglitazone in individuals after 3-6 month of treatment to ensure that only patients who are deriving benefit continue to be treated. Pioglitazone should be stopped in patients who do not respond adequately to treatment (e.g. reduction in Glycosylated haemoglobin HbA1C). • Before starting pioglitazone, the following known risk factors for development of bladder cancer should be assessed in individuals: age, current or past history of smoking, exposure to some occupational or chemotherapy agents such as cyclophosphamide, or previous irradiation of the pelvic region. • Use in elderly patients should be considered carefully before and during treatment because the risk of bladder cancer increases with age. Elderly patients should start on the lowest possible dose and be regularly monitored because of the risks of bladder cancer and heart failure associated with pioglitazone. COMPOSITION Each uncoated bilayered tablet contains: . 20 mg (InTeneligliptin Prolonged IP release .………………………..……………….. form) PioglitazoneMetformin Hydrochloride Hydrochloride IP IP …………………..…... equivalent to mg Pio . 15 mg .s Colour:glitazone Quinoline ………………………….…………………… Yellow Excipients ………………………….……………………..... q CLINICAL PHARMACOLOGY Biotenly®-MP contains three oral anti-hyperglycaemic drugs teneligliptin, pioglitazone and metformin hydrochloride used in the management of type-2 diabetes (NIDDM). MECHANISM OF ACTION/PHARMACODYNEMICS Teneligliptin is a DPP-4 inhibitor, which is believed to exert its actions in patients with type 2 diabetes by slowing the inactivation of incretin hormones. -

IJBCP International Journal of Basic & Clinical Pharmacology the Observational, Cross-Sectional Study of Drug Utilization
Print ISSN: 2319-2003 | Online ISSN: 2279-0780 IJBCP International Journal of Basic & Clinical Pharmacology DOI: http://dx.doi.org/10.18203/2319-2003.ijbcp20194771 Original Research Article The observational, cross-sectional study of drug utilization 90% and use of dipeptidyl peptidase-4 inhibitor in the patients with type 2 diabetes mellitus Prashant P. Shivgunde1*, Shantanu R. Joshi2, Archana D. Kodilkar1 1Department of University Research, Maharashtra ABSTRACT University of Health Sciences (MUHS), Mhasrul, Vani- Background: Diabetes is a chronic metabolic disease which affects the quality Dindori Road, Nashik, of life. It leads to multiple complications due to metabolic involvement. Out of Maharashtra, India multiple drugs used to treat diabetes, dipeptidyl peptidase 4 (DPP-4) inhibitors 2 Global Herbs Pharmaceuticals, are comparatively new drugs used for type-2-diabetes mellitus (DM) treatment. Pune-Satara Road, Pune, This study aimed to find out the drug utilization (DU) 90% and use of DPP-4 Maharashtra, India inhibitors in patients with type-2-DM. Methods: A prospective, cross-sectional, observational study was conducted at Received: 28 September 2019 a private healthcare clinic of an endocrinologist in Nashik. Type-2-DM patients Revised: 11 October 2019 of both sexes were selected and a total of 199 patients were enrolled in the Accepted: 14 October 2019 study. The consented patients were interviewed and prescription copies were collected. After studying them; statistical analysis was done and results and *Correspondence to: conclusions were drawn. Dr. Prashant P. Shivgunde, Results: Out of total prescribed drugs, 58.77% of drugs were anti-diabetics. It Email: prashantshivgunde@ was observed that the biguanides were most frequently (25.32%) prescribed gmail.com while the least prescribed drugs were meglitinide analogues (0.08%). -

202293Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 202293Orig1s000 RISK ASSESSMENT and RISK MITIGATION REVIEW(S) Department of Health and Human Services Public Health Service Food and Drug Administration Center for Drug Evaluation and Research Office of Surveillance and Epidemiology Office of Medication Error Prevention and Risk Management Final Risk Evaluation and Mitigation Strategy (REMS) Review Date: December 20, 2013 Reviewer(s): Amarilys Vega, M.D., M.P.H, Medical Officer Division of Risk Management (DRISK) Team Leader: Cynthia LaCivita, Pharm.D., Team Leader DRISK Drug Name(s): Dapagliflozin Therapeutic Class: Antihyperglycemic, SGLT2 Inhibitor Dosage and Route: 5 mg or 10 mg, oral tablet Application Type/Number: NDA 202293 Submission Number: Original, July 11, 2013; Sequence Number 0095 Applicant/sponsor: Bristol-Myers Squibb and AstraZeneca OSE RCM #: 2013-1639 and 2013-1637 *** This document contains proprietary and confidential information that should not be released to the public. *** Reference ID: 3426343 1 INTRODUCTION This review documents DRISK’s evaluation of the need for a risk evaluation and mitigation strategy (REMS) for dapagliflozin (NDA 202293). The proposed proprietary name is Forxiga. Bristol-Myers Squibb and AstraZeneca (BMS/AZ) are seeking approval for dapagliflozin as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus (T2DM). Bristol-Myers Squibb and AstraZeneca did not submit a REMS or risk management plan (RMP) with this application. At the time this review was completed, FDA’s review of this application was still ongoing. 1.1 BACKGROUND Dapagliflozin. Dapagliflozin is a potent, selective, and reversible inhibitor of the human renal sodium glucose cotransporter 2 (SGLT2), the major transporter responsible for renal glucose reabsorption. -
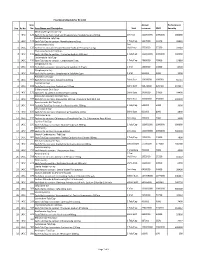
Sno. Rc No Item No. Item Name and Description Unit Annual Turnover
Final Drug Schedule for RC 145C Item Annual Performance Sno. Rc No No. Item Name and Description Unit turnover EMD Security Medroxy Progesterone Inj- 1 145C 126 Each ml to contain: Medroxy Progesterone Acetate Suspn.150mg. 1ml Vial 100000000 1000000 600000 Norethisterone Tab/Cap- 2 145C 128 Each Tab/Cap to contain: Norethisterone 5mg. 1 Tab/Cap 5637000 56370 33822 Dexamethasone Inj- 2ml 3 145C 133 Each ml to contain: Dexamethasone Sodium Phosphate 4 mg. Vial/Amp 5735000 57350 34410 Thyroxine Sodium Tab/Cap- 4 145C 135 Each Tab/Cap to contain: Thyroxine Sodium 100 mcg 1 Tab/Cap 100000000 1000000 600000 Carbimazole Tab/Cap- 5 145C 136 Each Tab/Cap to contain: Carbimazole 5 mg. 1 Tab/Cap 2980000 29800 17880 Streptomycin Inj- 6 145C 151 Each Vial to contain: Streptomycin Sulphate 0.75gm 1 Vial 1400000 14000 8400 Streptomycin Inj- 7 145C 152 Each Vial to contain: Streptomycin Sulphate 1gm 1 Vial 500000 5000 3000 Ampicillin Dry Syp- 8 145C 161 Each 5ml to contain: Ampicillin 125mg 30ml Bott 10959000 109590 65754 Cephalexin Syp- 9 145C 168 Each 5ml to contain: Cephalexin 125mg 30ml Bott 32874000 328740 197244 Erthyromycin Oral Susp- 10 145C 172 Each 5ml to contain: Erythromycin 125mg 30ml Bott 2400000 24000 14400 Amoxy & Clavulanic Acid Dry Syp- 11 145C 183 Each 5ml to contain: Amoxycillin 200 mg, Clavulanic Acid 28.5 mg 30ml Bott 35000000 350000 210000 Pyrazinamide Kid Tab/Cap- 12 145C 191 Each Kid Tab/Cap to contain: Pyrazinamide 300mg 1 Tab/Cap 500000 5000 3000 Chloroquine Syp- 13 145C 196 Each 5ml to contain: Chloroquine Phosphate 50mg. -

Meta-Analysis of 11 Heterogeneous Studies Regarding Dipeptidyl Peptidase 4 Inhibitor Add-On Therapy for Type 2 Diabetes Mellitus Patients Treated with Insulin
Hindawi Journal of Diabetes Research Volume 2020, Article ID 6321826, 12 pages https://doi.org/10.1155/2020/6321826 Research Article Meta-Analysis of 11 Heterogeneous Studies regarding Dipeptidyl Peptidase 4 Inhibitor Add-On Therapy for Type 2 Diabetes Mellitus Patients Treated with Insulin Katsuya Shibuki ,1,2 Shuji Shimada,1 and Takao Aoyama1 1Department of Pharmacy, Tokyo University of Science, 2641 Yamazaki, Noda 278-8510, Japan 2Clinical Research Center, Medical Hospital, Tokyo Medical and Dental University, 1-5-45 Yushima, Tokyo 113-8519, Japan Correspondence should be addressed to Katsuya Shibuki; [email protected] Received 6 April 2020; Revised 24 September 2020; Accepted 22 October 2020; Published 11 November 2020 Academic Editor: Daniela Foti Copyright © 2020 Katsuya Shibuki et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Background. Several clinical trials have addressed the therapeutic strategy of adding dipeptidyl peptidase 4 (DPP-4) inhibitors to the treatment of type 2 diabetes mellitus (DM) inadequately controlled by insulin therapy. However, there is a high degree of heterogeneity in these studies, and the cause of which has not been identified. Methods. We conducted a meta-analysis of randomized controlled trials, which compared the efficacy and safety of adding DPP-4 inhibitors or placebo to insulin therapy; the level of hemoglobin A1c (HbA1c) in the patients was >7.0%, and the duration of treatment was ≥8 weeks. We focused on the mean changes in HbA1c from the baseline (ΔHbA1c) and the incidence of hypoglycemia. -

Glomerular Hyperfiltration in Diabetes
BRIEF REVIEW www.jasn.org Glomerular Hyperfiltration in Diabetes: Mechanisms, Clinical Significance, and Treatment Lennart Tonneijck,* Marcel H.A. Muskiet,* Mark M. Smits,* Erik J. van Bommel,* † ‡ Hiddo J.L. Heerspink, Daniël H. van Raalte,* and Jaap A. Joles *Diabetes Center, Department of Internal Medicine, VU University Medical Center, Amsterdam, The Netherlands; †Department of Clinical Pharmacology, University Medical Center Groningen, Groningen, The Netherlands; and ‡Department of Nephrology and Hypertension, University Medical Center, Utrecht, The Netherlands ABSTRACT An absolute, supraphysiologic elevation in GFR is observed early in the natural characterized by an absolute, supraphy- history in 10%–67% and 6%–73% of patients with type 1 and type 2 diabetes, siologic increase in whole-kidney GFR respectively. Moreover, at the single-nephron level, diabetes-related renal hemo- (i.e., the sum of filtration in all function- dynamic alterations—as an adaptation to reduction in functional nephron mass and/ ing nephrons) (Figure 1). This early or in response to prevailing metabolic and (neuro)hormonal stimuli—increase glo- clinical entity, known as glomerular hy- merular hydraulic pressure and transcapillary convective flux of ultrafiltrate and perfiltration, is the resultant of obesity macromolecules. This phenomenon, known as glomerular hyperfiltration, classically and diabetes-induced changes in struc- has been hypothesized to predispose to irreversible nephron damage, thereby con- tural and dynamic factors that deter- tributing to initiation and progression of kidney disease in diabetes. However, ded- mine GFR.5 Reported prevalences of icated studies with appropriate diagnostic measures and clinically relevant end hyperfiltration at the whole-kidney level points are warranted to confirm this assumption. In this review, we summarize the vary greatly: between 10% and 67% in hitherto proposed mechanisms involved in diabetic hyperfiltration, focusing on type 1 diabetes mellitus (T1DM) (with ultrastructural, vascular, and tubular factors.