Bones Hold the Key to DNA Virus History and Epidemiology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Uh Fact Sheet University Bilateral.Pdf
The University of Helsinki is the Fact Sheet of the University of Helsinki oldest and largest institution of academic education in Finland, an University bilateral agreements, 2020-21 international scientific community of 40,000 students and researchers. In international university rankings, the University of Helsinki typically ranks among the top 100. The University of Helsinki seeks solutions for global challenges and creates new ways of thinking for the best of humanity. The University of Helsinki offers exchange students courses in 35 International Master's Programmes and in several Bachelor’s level programmes as well. Contact information International Exchange Services Address for courier post (FedEx, DHL, UPS etc.): P.O. Box 53 International Exchange Services, University of Helsinki FI-00014 University of Helsinki, Finland Fabianinkatu 28 (janitor's booth inside the courtyard) FI-00100 Helsinki, Finland Website: https://guide.student.helsinki.fi/en/exchange-and-visiting-students Student exchange: Email: [email protected] , Tel. +358 2941 22401 Teacher exchange: Email: [email protected] , Tel. +358 2941 40806, (Ms. Anne Rönkä) (more info in the end of this document) Agreement related issues: Email: [email protected] International education cooperation projects: Email: [email protected] (Ms. Anna Stina Sinisalo) Contact persons: Head of unit: Ms. Minna Koutaniemi, Overall coordination of student and teacher mobility Agreement and balance issues: Mr. Mikko Moilanen tel. +358 2941 22936 (Partner universities in the North America, Oceania, ISEP, N2N) Ms. AnneSophie Hokkanen, tel +358 2941 22178 (Partner universities in Africa) Ms. Mari Lauri, tel. +358 2941 22935 (Partner universities in Latin America) Ms. Raisa Asikainen, tel. +358 2941 22241 (Partner universities in Asia, Middle East and Europe) Incoming and outgoing students to/from Helsinki: Africa: Ms. -

Appraisal of Professor Markku KULMALA of the University of Helsinki for His Award Doctor Et Professor Honoris Causa from the Eötvös Loránd University
Appraisal of Professor Markku KULMALA of the University of Helsinki for his award Doctor et professor honoris causa from the Eötvös Loránd University Professor Markku Tapio Kulmala was born in 1958 in Finland. He graduated in 1983 and obtained his Ph.D. in 1988 both from the University of Helsinki. He has been the head of the Division of Atmospheric Sciences, Department of Physics of the University of Helsinki since 2001. In his nearest research environment, professor Kulmala leads a research group in Aerosol and Environmental Physics consisting of 60 people. He also leads the Finnish Centre of Excellence in Physics, Chemistry, Biology and Meteorology of Atmospheric Composition and Climate Change, which consists of 150 scientists. He was visiting professor at the University of Vienna and University of Lund, the King Carl XVI Gustaf’s visiting professor at the University of Stockholm. He is member of the editorial boards of several prestigious scientific journals. Professor Kulmala is a world leader in atmospheric aerosol science and one of the founders of terrestrial ecosystem meteorology. His major research interests cover the theory and mechanism of the new aerosol particle formation and growth processes in the atmosphere, their experimental studies, and the interactions among aerosol, clouds, climate and biosphere. Professor Kulmala has created a research program including continuous long-term atmospheric observations, global modelling and deep theoretical and experimental understanding of atmospheric cluster and aerosol dynamics. During his investigations, he has proposed that atmospheric clusters are crucial for new particle formation, and that they exist in the real atmosphere. He has shown that atmospheric nucleation takes place practically all around the world. -

Approved Unaffiliated Programs (Aups) 2022-2023 Terms Abroad
Approved Unaffiliated Programs (AUPs) 2022-2023 Terms Abroad Approved Unaffiliated Programs (AUPs) are pre-approved for transfer of credit back to the University of Denver. DU students must successfully complete the DU Study Abroad nomination process by indicated deadlines in order to receive approval to participate on an AUP. AUPs are approved based on the accredited institution issuing the transcript for student coursework abroad. In the case of some foreign institutions listed below, it may be possible for a student to enroll in the institution via a U.S. program provider (IES, CIEE, CEA, g-MEO, IGE, USAC, etc.); in doing so, it is the student's responsibility to verify that the program provider selected offers a transcript from the institution indicated below, otherwise DU may deny transfer of credit for the program. In the case of U.S. institutions that conduct their own programs abroad or serve as a school of record, transcripts from these institutions are approved ONLY for the study abroad program(s) indicated. Transcripts from these institutions for study abroad programs other than those indicated on the AUP list are not approved for transfer credit. Because of the fluid nature of health and safety abroad, all approved programs below may be reviewed by Risk Management after application and may not be approved based upon the security/health situation of the country or region. Study Abroad Program Transcript Institution Country Summer Only: Critical Language Scholarship Bryn Mawr College (SoR*) various School for Field Studies University -

2019 Annual Report Encompassing All Voices
2019 ANNUAL REPORT ENCOMPASSING ALL VOICES 2 Board member Wessel Meijer Radboud University Nijmegen, the Netherlands Board member President Sara Lopez Selga Sabine Pendl Vice-President Universitat Pompeu University of Michelle Stewart Board member Fabra, Spain Graz, Austria Michael Rosier University of University of Strathclyde, UK Hertfordshire, UK MESSAGE FROM THE EAIE BOARD 2019 was full of milestones and we have our wonderful EAIE volunteers, RAISING THE BAR office staff and community to thank for it. The EAIE’s activities and We started off 2019 in January with the launch of a brand new, knowledge creation focused on encompassing all voices to promote streamlined EAIE Awards portfolio to highlight outstanding contribution access, equity and inclusion in international higher education. Strategy in our community. The Spring Academy in Bordeaux kicked off a year development was a prominent point on the EAIE Leadership’s agenda of successful training events, that was followed up by webinars that sold throughout the year. Leaders came together for a series of highly out within 24 hours. This was further topped by the Autumn Academy collaborative workshops and engaged with the wider community to in Budapest, that proved to be our most international and well-attended develop the new EAIE strategy which will come into effect in September academy to date. In September, our 31st Annual Conference and 2020. Moreover, our current strategic goals to inspire professionals, be Exhibition in Helsinki, Finland was the largest event to date with 6222 their voice, strengthen our community and remain relevant, motivated us participants from 95 countries. to ensure that our work yielded positive impacts on a variety of levels. -

Mapping the Future of Law Agenda
Mapping the Future of Law Una Europa Virtual Seminar June 17-18, 2021, at 13:00h – 17:00h CET Registration: https://forms.office.com/r/FRTF4iBHhJ The Future Law Lab of Jagiellonian University in Krakow, Poland, cordially invites you for a two-day short, virtual seminar titled “Mapping the Future of Law.” The event aims to facilitate dialogue between established scholars and junior researchers from the universities making up the Una Europa Alliance.1 We want to create a space of mutual learning, enabling the participants to share their present work, take a glimpse at the frontier of research in areas other than their own, and allow new ideas to spark and grow. We can’t predict the future, but we can try to steer it in certain directions. To do so, however, we need a firm understanding of the current trends shaping our societies. Digitalization is one of them. With more spheres of life moving online, the world is getting smaller, yet inequalities are growing. Unprecedented amounts of data are generated, leading to both good and malicious uses of the new technologies. As certain old centers of power wither, new ones emerge. The law plays a role in causing these transformations and has a role to play in making sure that the weakest among us are not harmed while the benefits are justly shared. All these rapid socio-technological changes affect legal scholarship. On the one hand, new kinds of questions need answering. On the other, new methods of addressing them become available. And each one of us is a participant-observer in these changes. -

The University of Helsinki Annual Review 2019
ANNUAL REVIEW 2019 503 DOCTORAL DEGREES UNIVERSITY OF HELSINKI - ANNUAL REVIEW 2019 UNIVERSITY OF HELSINKI ANNUAL REVIEW 2019 Published 30 Apr 2020 Editorial board: Päivi Kuuppelomäki Jonas Lindholm Tuula Sunnarborg Soile Tapio Translation: University of Helsinki Language Services UNIVERSITY OF HELSINKI - ANNUAL REVIEW 2019 INTRODUCTION BY THE RECTOR LOOKING FORWARD TO NEW INITIATIVES AS WE HEAD TOWARDS 2030 The year 2019 was in many ways significant for the to lobby for sufficient funding for these important is- future of the University of Helsinki. The whole Uni- sues. versity community joined forces in drafting our stra- tegic plan for the next 10 years, and we also lobbied The year 2019 also saw the formulation of our new for university funding through the #Researchmat- strategic plan. Unlike the University’s previous stra- ters campaign. tegic plans, the new one spans 10 years, which means that we are envisioning the future all the way up to The entire Finnish university sector eagerly waited 2030. for the publication of the new Government pro- gramme in the spring of 2019 as well as for the re- The most important aspect in the year-long endeav- sults of the budget negotiations that took place the our was that the whole University community had following autumn. However, instead of just waiting the opportunity to participate and have a say in the for things to happen, we made things happen. Since strategic choices to be documented in the plan. Dur- 2018, the University of Helsinki has been a commit- ing this year, the University community was invited ted participant in the #Researchmatters campaign to respond to an online survey in January and Febru- with the aim of defending Bildung and enhancing ary, engage in face-to-face dialogue with the rector high-quality academic education, the appreciation of and vice-rectors in open campus meetings in April, science and funding for the higher education sector. -

2007 Final List of Participants
1 2007 ICES Annual Science Conference 17–21 September Helsinki, Finland Final list of participants Name, institute, country, and e-mail address Albert, Ole Thomas Institute of Marine Research A Lebre, M. Manuela Norway IPIMAR E-mail: [email protected] Portugal E-mail: [email protected] Aleksandrov, Sergey AtlantNIRO Aalto, Annika Russia University of Helsinki E-mail: [email protected] Finland E-mail: [email protected] Alekseevi, Irina IHF, Uni. Hamburg Abbors, Tom Germany Uudenmaan TE-keskus E-mail: [email protected] Finland E-mali : [email protected] Alenius, Pekka Finnish Institute of Marine Research Acuna, Enzo Finland Universidad Catolica del Norte - E-mail: [email protected] Sede Coquimbo Chile Alheit, Jürgen E-mail: [email protected] Baltic Sea Research Institute Germany Addison, Julian E-mail: [email protected] Centre for Environment Fisheries and Aquaculture Science Ameryk, Anetta United Kingdom Sea Fisheries Institute Gdynia E-mail: [email protected] Poland E-mail: [email protected] Aho, Teija Swedish Board of Fisheries Amiard-Triquet, Claude Institute of Coastal Research CNRS (Centre National de la Sweden Recherche Scientifique, France) E-mail: [email protected] France E-mail: [email protected] Ahvonen, Anssi Finnsh Game and Fisheries Research Inst. Amundrud, Trisha Finland Fisheries Research Services E-mail: [email protected] United Kingdom E-mail: [email protected] Akkanen, Jarkko University of Joensuu Anderson, Emory Finland Retired E-mail: [email protected] -

First Name Family Name Institution Country 1 Emil Airta University Of
Contemporary Analysis and Its Applications 8ECM Satellite Conference June 16-18, 2021 List of participants First Name Family Name Institution Country 1 Emil Airta University of Helsinki Finland 2 Edoardo Angeloni Iti Teramo Italy 3 Jean-Philippe ANKER University of Orleans France 4 NICOLA ARCOZZI University of Bologna Italia 5 Kari Astala University of Helsinki Finland 6 Francesca Astengo Università di Genova Italy 7 Olena Atlasiuk Institute of Mathematics of the National Academy of Sciences of Ukraine Ukraine 8 Rami Ayoush EIMI Russia 9 Annalisa Baldi University of Bologna Italy 10 Siegfried Beckus University of Potsdam Germany 11 Jonathan Bennett University of Birmingham UK 12 Gregory Berkolaiko Texas A&M University USA 13 Jerry Bona University of Illinois at Chicago United States 14 MUSTAPHA BOUALLALA faculté polydisciplinaire de safi, Université Cadi Ayyad Morocco 15 Roberto Bramati Politecnico di Torino Italy 16 Gianmarco Brocchi University of Birmingham United Kingdom 17 Aleksandar Bulj University of Zagreb Croatia 18 Gennadiy Burlak Universidad Autonoma del Estado de Morelos, CIICAp Mexico 19 Mattia Calzi Università degli Studi di Milano Italy 20 Tony Carbery University of Edinburgh Scotland 21 Andrea Carbonaro University of Genoa Italy 22 Valentina Casarino Università degli Studi di Padova Italia 23 Alessandra Cauli Politecnico Italy 24 Leonid Chaichenets TU Dresden Germany 25 Nikolaos Chalmoukis University of Bologna Italy 26 Andreia Chapouto University of Edinburgh United Kingdom 27 Thomas Chen Academy for Mathematics, Science, -

OCR Document
Fact Sheet of the University of Helsinki Erasmus+ Global Mobility (ICM), 2020-21 The University of Helsinki is the oldest and largest institution of academic education in Finland, an international scientific community of 40,000 students and researchers. In international university rankings, the University of Helsinki typically ranks among the top 100. The University of Helsinki seeks solutions for global challenges and creates new ways of thinking for the best of humanity. Contact information Erasmus code SF HELSINK01 ECHE 29604 PIC 999994535 Coordinating unit International Exchange Services Address for courier post (FedEx, DHL, UPS etc.): for university level P.O. Box 53 International Exchange Services, University of Helsinki student and FI-00014 University of Helsinki, Finland Fabianinkatu 28 (janitor's booth inside the courtyard) teacher exchange FI-00100 Helsinki, Finland Website: https://guide.student.helsinki.fi/en/exchange-and-visiting-students Student exchange: [email protected] Tel. +358 2941 22401 Staff exchange: [email protected] Tel: +358 2941 40806 Agreements: [email protected] International education cooperation projects: [email protected] Nominations Student exchange: Partners are asked to send nominations through an electronic form: https://elomake.helsinki.fi/lomakkeet/80266/lomake.html We do not have a separate deadline for nominations, but we recommend that students are nominated at least two weeks before the application deadlines. An automatic email with application instructions is sent to nominated students right after nomination. Teacher exchange (if included in the agreement) Nominations should be sent through an electronic form: https://elomake.helsinki.fi/lomakkeet/95196/lomake.html at least eight weeks before the estimated arrival date. -

Research Collaboration Guide.Indd
Acknowledgements Interviewees Prof. Agnieszka Rothert, University of Warsaw Prof. Kers n Stahl, University of Freiburg Prof. Andrew Patrizio, University of Edinburgh Dr. Kevin Mitchell, Trinity College Dublin Dr. Anniek de Ruijter, University of Amsterdam Prof. Dr. Laura Bieger, University of Groningen Prof. Dr. Burkhard Becher, University of Zurich Dr. Leonardo Tondo, Harvard University Prof. Carol Brayne, University of Cambridge Dr. Makoto Miyara, Sorbonne University Prof. Catherine Lyall, University of Edinburgh Dr. Malu Ga o, University College London Dr. Charlo e Ribeyrol, Sorbonne University Prof. Mario Dell’Agli, University of Milan Dr. Cris na Staub, Service Sans Soucis Prof. Mark Rehkämper, Imperial College London Dr. Elio Shijaku, University of Barcelona Dr. Marta Costa, University of Cambridge Prof. Dr. Els Stronks, Utrecht University Prof. Michele Vendruscolo, University of Cambridge Dr. Esteve Fernandez, University of Barcelona Dr. Nici Zimmermann, Univeristy College London Dr. Eszter Voroshazi, IMEC Dr. Nollaig Bourke, Trinity College Dublin Dr. Frédéric Suff ert, Ins tut Na onal de la Recherche Dr. Olivier Schwander, Sorbonne university Agronomique) Prof. Sir Peng Tee Khaw, Univeristy College London Prof. Fredrik Tufvesson, Lund University Prof. Pete Nellist, University of Oxford Prof. Guda van Noort, University of Amsterdam Dr. Rebecca Brauchli, University of Zurich Dr. Isabel Fletcher, University of Edinburgh Dr. Ross Puves, University of Zurich Dr. James Tufano, Charles University in Prague Prof. Sampsa Hautaniemi, University of Helsinki Dr. Jan Šnajder, University of Zagreb Dr. Sara Sa n, University of Milan Prof. Jane Ohlmeyer, Trinity College Dublin Dr. Simon Smith, University of Edinburgh Dr. Janko Jankovic, University of Belgrade Prof. Stéphanie Henne e-Vauchez, University Paris Prof. -
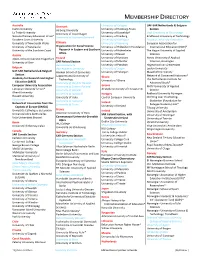
Membership Directory
MEMBERSHIP DIRECTORY Australia Denmark University of Cologne UAF-SAR Netherlands & Belgium Curtin University Aalborg University University of Duisburg-Essen Section La Trobe University University of Copenhagen University of Düsseldorf Delft University of Technology National Tertiary Education Union* University of Southern Denmark University of Freiburg Eindhoven University of Technology Southern Cross University University of Göttingen Erasmus University Rotterdam University of New South Wales Ethiopia University of Hamburg European Association for University of Newcastle Organization for Social Science University of Hildesheim Foundation International Education (EAIE)* University of the Sunshine Coast Research in Eastern and Southern University of Hohenheim The Hague University of Applied Africa Austria University of Kassel Sciences Alpen-Adria-Universität Klagenfurt Finland University of Konstanz Hanze University of Applied University of Graz SAR Finland Section University of Münster Sciences, Groningen Aalto University University of Potsdam Hogeschool van Amsterdam Belgium Åbo Akademi University University of Siegen Leiden University UAF-SAR Netherlands & Belgium Hanken School of Economics University of Tübingen Maastricht University Section Lappeenranta University of Network of Concerned Historians* Academy for Research and Higher Ghana Technology University of Ghana The Netherlands Institute for Education (ARES) University of the Arts Helsinki Advanced Study* European University Association University of Eastern Finland Greece NHTV -

University of Helsinki Fact Sheet for Incoming Exchange Students In
The University of Helsinki is the Fact Sheet of the University of Helsinki oldest and largest institution of academic education in Finland, an Faculty and unit bilateral agreements, 2020-21 international scientific community Faculty of Law of 40,000 students and researchers. In international university rankings, the University of Helsinki typically ranks among the top 100. The University of Helsinki seeks solutions for global challenges and creates new ways of thinking for the best of humanity. The University of Helsinki offers exchange students courses in 35 International Master's Programmes and in several Bachelor’s level programmes as well. Contact information Coordinating unit International Exchange Services Address for courier post (FedEx, DHL, UPS etc.): for university level P.O. Box 53 International Exchange Services, University of Helsinki student and FI-00014 University of Helsinki, Finland Fabianinkatu 28 (janitor's booth inside the courtyard) teacher exchange FI-00100 Helsinki, Finland Website: https://guide.student.helsinki.fi/en/exchange-and-visiting-students Agreement related issues: [email protected] International education cooperation projects: [email protected] Contact person for Ms. AnneSophie Hokkanen faculty/unit level Student Exchange Specialist student exchanges Tel. +358 2941 22401 [email protected] Contact person for Ms. Anne Rönkä teacher exchange Teacher, Researcher and Staff Exchange Specialist (if teacher exchange is Help with accommodation arrangements and other practicalities