Case Studies Controversies in Vaccination
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Hexavalent Dtap/IPV/Hib/Hepb Combination Vaccine: Information for Healthcare Practitioners About the Neonatal Selective
The hexavalent DTaP/IPV/Hib/HepB combination vaccine Information for healthcare practitioners about the neonatal selective immunisation programme for babies at risk of hepatitis B The Hexavalent DTaP/IPV/Hib/HepB combination vaccine: Information for Healthcare Practitioners (selective programme) About Public Health England Public Health England exists to protect and improve the nation’s health and wellbeing, and reduce health inequalities. We do this through world-leading science, research, knowledge and intelligence, advocacy, partnerships and the delivery of specialist public health services. We are an executive agency of the Department of Health and Social Care, and a distinct delivery organisation with operational autonomy. We provide government, local government, the NHS, Parliament, industry and the public with evidence-based professional, scientific and delivery expertise and support. Public Health England Wellington House 133-155 Waterloo Road London SE1 8UG Tel: 020 7654 8000 www.gov.uk/phe Twitter: @PHE_uk Facebook: www.facebook.com/PublicHealthEngland For queries relating to this document, please contact: [email protected] © Crown copyright 2020 You may re-use this information (excluding logos) free of charge in any format or medium, under the terms of the Open Government Licence v3.0. To view this licence, visit OGL. Where we have identified any third party copyright information you will need to obtain permission from the copyright holders concerned. First published November 2017 This updated version published February -

Download File (Pdf)
2021 FORUM REPORT COVID-19 in Africa one year on: Impact and Prospects MO IBRAHIM FOUNDATION 2021 FORUM REPORT COVID-19 in Africa one year on: Impact and Prospects MO IBRAHIM FOUNDATION Foreword by Mo Ibrahim Notwithstanding these measures, on current projections Founder and Chair of the Mo Ibrahim Africa might not be adequately covered before 2023. Foundation (MIF) Vaccinating Africa is an urgent matter of global security and all the generous commitments made by Africa’s partners must now be delivered. Looking ahead - and inevitably there will be future pandemics - Africa needs to significantly enhance its Over a year ago, the emergence and the spread of COVID-19 homegrown vaccine manufacturing capacity. shook the world and changed life as we knew it. Planes were Africa’s progress towards its development agendas was off grounded, borders were closed, cities were shut down and course even before COVID-19 hit and recent events have people were told to stay at home. Other regions were hit created new setbacks for human development. With very earlier and harder, but Africa has not been spared from the limited access to remote learning, Africa’s youth missed out pandemic and its impact. on seven months of schooling. Women and girls especially The 2021 Ibrahim Forum Report provides a comprehensive are facing increased vulnerabilities, including rising gender- analysis of this impact from the perspectives of health, based violence. society, politics, and economics. Informed by the latest data, The strong economic and social impacts of the pandemic it sets out the challenges exposed by the pandemic and the are likely to create new triggers for instability and insecurity. -

(ACIP) General Best Guidance for Immunization
8. Altered Immunocompetence Updates This section incorporates general content from the Infectious Diseases Society of America policy statement, 2013 IDSA Clinical Practice Guideline for Vaccination of the Immunocompromised Host (1), to which CDC provided input in November 2011. The evidence supporting this guidance is based on expert opinion and arrived at by consensus. General Principles Altered immunocompetence, a term often used synonymously with immunosuppression, immunodeficiency, and immunocompromise, can be classified as primary or secondary. Primary immunodeficiencies generally are inherited and include conditions defined by an inherent absence or quantitative deficiency of cellular, humoral, or both components that provide immunity. Examples include congenital immunodeficiency diseases such as X- linked agammaglobulinemia, SCID, and chronic granulomatous disease. Secondary immunodeficiency is acquired and is defined by loss or qualitative deficiency in cellular or humoral immune components that occurs as a result of a disease process or its therapy. Examples of secondary immunodeficiency include HIV infection, hematopoietic malignancies, treatment with radiation, and treatment with immunosuppressive drugs. The degree to which immunosuppressive drugs cause clinically significant immunodeficiency generally is dose related and varies by drug. Primary and secondary immunodeficiencies might include a combination of deficits in both cellular and humoral immunity. Certain conditions like asplenia and chronic renal disease also can cause altered immunocompetence. Determination of altered immunocompetence is important to the vaccine provider because incidence or severity of some vaccine-preventable diseases is higher in persons with altered immunocompetence; therefore, certain vaccines (e.g., inactivated influenza vaccine, pneumococcal vaccines) are recommended specifically for persons with these diseases (2,3). Administration of live vaccines might need to be deferred until immune function has improved. -

UNICEF Pentavalent-Hexa 2021+ Pre-Tender Industry Consultation
UNICEF Pentavalent-Hexa 2021+ Pre-Tender Industry Consultation 19th SEPTEMBER 2019 1 Presentation Outline 1. Context ▪ Gavi Board decision ▪ Hexa strategic alignment ▪ Value Based Assessment 2. Demand scenarios and assumptions 3. Pentavalent Tender: ▪ Objectives ▪ Scope ▪ Duration ▪ Modality 4. Summary of feedback and response to questionnaire 5. Timelines 1. CONTEXT Gavi Board decision Hexa strategic alignment Value Based Assessment 3 Gavi Board November 2018 – Decisions IPV Post-2020 DTP booster VIS 2018 VIS 2018 + Hexavalent as immunisation option • Approved support for D, T & P vaccines (Td, DTwP, pentavalent) to be used as booster doses beginning in 2021 • Approved support for inactivated poliovirus vaccine (IPV), with country financing arrangements subject to alignment with the final parameter setting for Gavi 5.0 at the June 2019 Board meeting* • Approved in principle support of wP-Hexavalent vaccine, subject to a vaccine being licenced, recommended for use by WHO, WHO pre-qualified and that market attributes support the successful implementation of Hexavalent The development of capacity for standalone IPV remains the main priority for Gavi Alliance as part of the effort to eradicate polio All Gavi Board decisions are “subject to the availability of funding for the 2021-2025 period and alignment with the final parameter setting for Gavi 5.0 at the June 2019 Board meeting.” *Gavi's Board approved IPV co-financing arrangements post-2020 in its June 2019 session 4 Conditions to open a funding window for Hexavalent Condition 1: Hexavalent vaccines that are candidates for Gavi support should achieve IPV immunogenicity targets as per WHO’s SAGE recommendations. Condition 2: Hexavalent vaccines are priced in line with value-based principles. -

Candidate Rotavirus Vaccine Recommendations for Consideration by the WHO Strategic Advisory Group of Experts (SAGE) on Immunization
Candidate rotavirus vaccine recommendations for consideration by the WHO Strategic Advisory Group of Experts (SAGE) on Immunization 1. Overall recommendation WHO strongly recommends the inclusion of rotavirus vaccination into the national immunization programmes of all regions of the world. In particular, countries where deaths among children due to diarrhoeal diseases account for ≥10% of under-5 mortality rate should prioritize the introduction of rotavirus vaccination. Countries where deaths among children due to diarrhoeal diseases account for <10% of under-5 mortality rate should also consider the introduction of rotavirus vaccination based on anticipated reduction in mortality and morbidity from diarrhoea, savings in health care costs, and the cost-effectiveness of vaccination. Justification: Rotavirus is a major cause of mortality in countries with high diarrhoeal disease mortality among children under five years of age. Every year, rotavirus gastroenteritis is estimated to cause approximately 527,000 (475,000-580,000) deaths globally among children <5 years old. Most of these deaths occur in developing countries and 90% of the rotavirus- associated fatalities occur in Africa and Asia alone. Globally, >2 million children are hospitalized each year for rotavirus infections. In a recent report of sentinel hospital-based rotavirus surveillance from 35 nations representing each of the six WHO regions between 2001 and 2008, an average of 40% (range= 34%-45%) of hospitalizations for diarrhea among children < 5 years old were attributable to rotavirus infection. 2. Detailed recommendation: Extrapolating efficacy data from a rotavirus vaccine study performed in one population to use of same rotavirus vaccine in other populations Efficacy/effectiveness data from a rotavirus vaccine study performed in a population from one of three under-5 mortality rate categories* can be extrapolated for use in populations in the same under-5 mortality rate category. -

Rotavirus Vaccine: Questions and Answers for Health Care Providers
Rotavirus Vaccine Questions and Answers for Health Care Providers In April 2014, Manitoba Health, Seniors and Active Living launched a publicly-funded Rotavirus Immunization Program for infants born on or after March 1, 2014. In 2018, Manitoba, along with the rest of Canada, switched from RotarixTM to RotaTeq®. As of May 15, 2021, Manitoba has switched back to Rotarix®, for use in its publicly-funded Rotavirus Immunization Program, for infants born on or after April 1, 2021. This document includes an updated list of questions and answers for your reference. 1. Why is there a Rotavirus Immunization Program in Manitoba? 2. Who qualifies for publicly-funded rotavirus vaccine? 3. Which rotavirus vaccine does Manitoba use? 4. Why does the vaccine series need to be completed before eight months of age? 5. How is Rotarix® packaged? 6. How are the oral tube and cap disposed of after use? 7. Should a spit-up dose of vaccine be repeated? 8. Are there any precautions that health care providers should take when administering the oral rotavirus vaccine? 9. Oral rotavirus vaccine contains sucrose in an amount expected to have an effect on immunization injection pain. When should Rotarix® be given in relation to other vaccines to elicit a reduction in pain? 10. How do I administer Rotarix®? 11. Is additional screening for potential contraindications required prior to administering rotavirus vaccine? 12. Can infants born to mothers on immunosuppressive medication be immunized? 13. Are there any issues related to circulating maternal antibodies interfering with the response to the live attenuated vaccine? 14. Are the two rotavirus vaccines, RotaTeq® and Rotarix™, interchangeable? 15. -

Immunogenicity and Safety of a Fully Liquid Dtap-IPV-Hep B-PRP-T
ccines & a V f V a o c l c i a n n a Lanata et al., J Vaccines Vaccin 2012, 3:1 r t u i o o n J Journal of Vaccines & Vaccination DOI: 10.4172/2157-7560.1000128 ISSN: 2157-7560 Research Article Open Access Immunogenicity and Safety of a Fully Liquid DTaP-IPV-Hep B-PRP-T Vaccine at 2-4-6 Months of Age in Peru Claudio Lanata1, Betzana Zambrano2, Lucie Ecker1, Isabel Amemiya1, Ana Gil1 and Eduardo Santos Lima3* 1Instituto de Investigación Nutricional, Lima, Peru 2Sanofi Pasteur, Montevideo, Uruguay 3Clinical Development, Sanofi Pasteur, Lyon, France Abstract Objectives: To assess the immunogenicity and safety of a new candidate, fully liquid, hexavalent DTaP-IPV- Hep B-PRP-T vaccine (Hexaxim™, an AcXim family vaccine) compared to a licensed hexavalent DTaP-IPV-Hep B// PRP-T vaccine (Infanrix hexa®) in Peru. Methods: Infants born to HBsAg seronegative mothers and who had not received a hepatitis B vaccine prior to entry into the study were randomized to receive either Hexaxim™ (Group 1) or Infanrix hexa® (Group 2) at 2, 4, 6 months of age. Seroprotection (SP) rate for hepatitis B (anti-HBs antibody concentration ≥10 mIU/mL) was analysed for non-inferiority (Group 1 minus Group 2) 1 month post-primary series. Anti-diphtheria and anti-polyrosil ribitol phosphate (PRP) antibody responses were analysed descriptively. Safety was analysed from parental reports. Results: Seroprotection rate for anti-HBs antibody titers ≥10 mIU/mL was high in both groups (≥99.2%) and non-inferiority was demonstrated (lower bound of the 95% CI for the difference was -4.17, above the pre-defined delta [-10%]). -

Pink Book Webinar Series: Rotavirus and Hepatitis a Slides
Centers for Disease Control and Prevention National Center for Immunization and Respiratory Diseases Rotavirus and Hepatitis A Pink Book Webinar Series 2018 Mark Freedman, DVM, MPH Veterinary Medical Officer Photographs and images included in this presentation are licensed solely for CDC/NCIRD online and presentation use. No rights are implied or extended for use in printing or any use by other CDC CIOs or any external audiences. Rotavirus: Disease and Vaccine Rotavirus . First identified as a cause of diarrhea in 1973 . Most common cause of severe gastroenteritis in infants and young children . Nearly universal infection by age 5 years . Responsible for up to 500,000 diarrheal deaths each year worldwide Rotavirus . Two important outer shell proteins—VP7, or G-protein, and VP4, or P-protein define the serotype of the virus . From 1996–2005, five predominate strains in the U.S. (G1–G4, G9) accounted for 90% of the isolates . G1 strain accounts for 75% of infections . Very stable and may remain viable for weeks or months if not disinfected Rotavirus Immunity . Antibody against VP7 and VP4 probably important for protection • Cell-mediated immunity probably plays a role in recovery and immunity . First infection usually does not lead to permanent immunity . Reinfection can occur at any age . Subsequent infections generally less severe Rotavirus Clinical Features . Short incubation period . First infection after 3 months of age generally most severe . May be asymptomatic or result in severe, dehydrating diarrhea with fever and vomiting . Gastrointestinal symptoms generally resolve in 3–7 days Rotavirus Complications . Infection can lead to severe diarrhea, dehydration, electrolyte imbalance, and metabolic acidosis . -

Detailed Review Paper on Rotavirus Vaccines
Rotavirus Vaccines 17 March 2009 Detailed Review Paper on Rotavirus Vaccines To be presented to the WHO Strategic Advisory Group of Experts (SAGE) on Immunization, April 2009 Ad-hoc group of experts on rotavirus vaccines Chair : G. Peter Members: T. Aguado, Z. Bhutta, L. De Oliveira, K. Neuzil, U. Parashar, D. Steele WHO Secretariat: C. Mantel, S. Wang, G. Mayers, E. Derobert Rapporteur: D. Payne 1 Rotavirus Vaccines 17 March 2009 Table of Contents I. Rotavirus Epidemiology and Rationale for Vaccination 1. Disease burden 2. Rationale for vaccination as the primary preventive measure II. Rotavirus Vaccine Efficacy and Safety in Pivotal Pre-Licensure Trials Brief summary of rotavirus vaccines 1. Rotarix ® 2. RotaTeq ® III. Newly Available Data from Clinical Trials in Africa and Asia and Post-introduction Vaccine Effectiveness Evaluations in the Americas 1. South Africa and Malawi clinical trials (Rotarix ®) 2. Hong Kong, Taiwan, and Singapore clinical trials (Rotarix ®) 3. Nicaragua post-introduction vaccine effectiveness case- control study (RotaTeq ®) 4. El Salvador post-introduction vaccine effectiveness case- control study (Rotarix ®) 5. United States post-licensure impact evaluation studies 6. Status of other ongoing studies IV. Vaccine Safety, Co-Administration, and Special Populations 1. Vaccine safety 2. Co-administration with other vaccines, particularly OPV 3. HIV-infected populations 4. Breast-feeding and Pre-term Infants V. Vaccine Schedules and Age Restrictions VI. Vaccine Cost-effectiveness and Decision-Making Regarding Program Implementation 1. Cost-effectiveness and affordability 2. Decision-making regarding vaccine introduction VII. Vaccine Program Implementation and Vaccine Delivery Logistics VIII. Integration with Diarrheal Control and Other Health Interventions and Communication 1. -

(Pneumococcal Conjugate Vaccine) Schedule
Changes to the infant pneumococcal conjugate vaccine schedule Information for healthcare practitioners Changes to the infant pneumococcal conjugate vaccine schedule About Public Health England Public Health England exists to protect and improve the nation’s health and wellbeing, and reduce health inequalities. We do this through world-leading science, research, knowledge and intelligence, advocacy, partnerships and the delivery of specialist public health services. We are an executive agency of the Department of Health and Social Care, and a distinct delivery organisation with operational autonomy. We provide government, local government, the NHS, Parliament, industry and the public with evidence-based professional, scientific and delivery expertise and support. Public Health England Wellington House 133-155 Waterloo Road London SE1 8UG Tel: 020 7654 8000 www.gov.uk/phe Twitter: @PHE_uk Facebook: www.facebook.com/PublicHealthEngland For queries relating to this document, please contact: [email protected] © Crown copyright 2019 You may re-use this information (excluding logos) free of charge in any format or medium, under the terms of the Open Government Licence v3.0. To view this licence, visit OGL. Where we have identified any third party copyright information you will need to obtain permission from the copyright holders concerned. Published December 2019 PHE publications PHE supports the UN gateway number: 2019204 Sustainable Development Goals 2 Changes to the infant pneumococcal conjugate vaccine schedule Contents About -
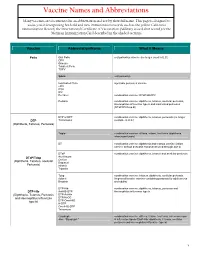
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many Vaccines Are Documented in an Abbreviation and Not by Their Full Name
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many vaccines are documented in an abbreviation and not by their full name. This page is designed to assist you in interpreting both old and new immunization records such as the yellow California Immunization Record, the International Certificate of Vaccination (Military issued shot record) or the Mexican Immunization Card described in the shaded sections. Vaccine Abbreviation/Name What it Means Polio Oral Polio oral poliovirus vaccine (no longer used in U.S.) OPV Orimune Trivalent Polio TOPV Sabin oral poliovirus Inactivated Polio injectable poliovirus vaccine eIPV IPOL IPV Pentacel combination vaccine: DTaP/Hib/IPV Pediarix combination vaccine: diphtheria, tetanus, acellular pertussis, Haemophilus influenzae type b and inactivated poliovirus (DTaP/IPV/Hep B) DTP or DPT combination vaccine: diphtheria, tetanus, pertussis (no longer DTP Tri-Immunol available in U.S.) (Diphtheria, Tetanus, Pertussis) Triple combination vaccine: difteria, tétano, tos ferina (diphtheria, tetanus pertussis) DT combination vaccine: diphtheria and tetanus vaccine (infant vaccine without pertussis component used through age 6) DTaP combination vaccine: diphtheria, tetanus and acellular pertussis DTaP/Tdap Acel-Imune Certiva (Diphtheria, Tetanus, acellular Daptacel Pertussis) Infanrix Tripedia Tdap combination vaccine: tetanus, diphtheria, acellular pertussis Adacel (improved booster vaccine containing pertussis for adolescents Boostrix and adults) DTP-Hib combination vaccine: diphtheria, tetanus, -

(Nitags) and WHO Immunisation-Related Advisory Committees
Summary of recent issues considered by four national immunisation technical advisory groups (NITAGs) and WHO immunisation-related advisory committees Prepared by the National Centre for Immunisation Research & Surveillance (NCIRS) Period of review: 16/05/2019 to 13/09/2019 Contents 1 Advisory Committee on Immunization Practices (ACIP), USA .......................................................... 3 1.1 ACIP meeting: 26-27 June 2019 ....................................................................................................... 3 1.2 Newly published or updated recommendations ............................................................................... 20 1.2.1 Japanese Encephalitis Vaccine: Recommendations of the Advisory Committee on Immunisation Practices.......................................................................................................................... 20 1.2.2 Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practice ..................................................................................................... 20 1.2.3 Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2019–20 Influenza Season ............ 21 1.3 New or updated recommendations – not yet published ................................................................... 21 2 Immunisation Advisory Centre (IMAC), New Zealand ....................................................................