(Pneumococcal Conjugate Vaccine) Schedule
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(ACIP) General Best Guidance for Immunization
8. Altered Immunocompetence Updates This section incorporates general content from the Infectious Diseases Society of America policy statement, 2013 IDSA Clinical Practice Guideline for Vaccination of the Immunocompromised Host (1), to which CDC provided input in November 2011. The evidence supporting this guidance is based on expert opinion and arrived at by consensus. General Principles Altered immunocompetence, a term often used synonymously with immunosuppression, immunodeficiency, and immunocompromise, can be classified as primary or secondary. Primary immunodeficiencies generally are inherited and include conditions defined by an inherent absence or quantitative deficiency of cellular, humoral, or both components that provide immunity. Examples include congenital immunodeficiency diseases such as X- linked agammaglobulinemia, SCID, and chronic granulomatous disease. Secondary immunodeficiency is acquired and is defined by loss or qualitative deficiency in cellular or humoral immune components that occurs as a result of a disease process or its therapy. Examples of secondary immunodeficiency include HIV infection, hematopoietic malignancies, treatment with radiation, and treatment with immunosuppressive drugs. The degree to which immunosuppressive drugs cause clinically significant immunodeficiency generally is dose related and varies by drug. Primary and secondary immunodeficiencies might include a combination of deficits in both cellular and humoral immunity. Certain conditions like asplenia and chronic renal disease also can cause altered immunocompetence. Determination of altered immunocompetence is important to the vaccine provider because incidence or severity of some vaccine-preventable diseases is higher in persons with altered immunocompetence; therefore, certain vaccines (e.g., inactivated influenza vaccine, pneumococcal vaccines) are recommended specifically for persons with these diseases (2,3). Administration of live vaccines might need to be deferred until immune function has improved. -

Candidate Rotavirus Vaccine Recommendations for Consideration by the WHO Strategic Advisory Group of Experts (SAGE) on Immunization
Candidate rotavirus vaccine recommendations for consideration by the WHO Strategic Advisory Group of Experts (SAGE) on Immunization 1. Overall recommendation WHO strongly recommends the inclusion of rotavirus vaccination into the national immunization programmes of all regions of the world. In particular, countries where deaths among children due to diarrhoeal diseases account for ≥10% of under-5 mortality rate should prioritize the introduction of rotavirus vaccination. Countries where deaths among children due to diarrhoeal diseases account for <10% of under-5 mortality rate should also consider the introduction of rotavirus vaccination based on anticipated reduction in mortality and morbidity from diarrhoea, savings in health care costs, and the cost-effectiveness of vaccination. Justification: Rotavirus is a major cause of mortality in countries with high diarrhoeal disease mortality among children under five years of age. Every year, rotavirus gastroenteritis is estimated to cause approximately 527,000 (475,000-580,000) deaths globally among children <5 years old. Most of these deaths occur in developing countries and 90% of the rotavirus- associated fatalities occur in Africa and Asia alone. Globally, >2 million children are hospitalized each year for rotavirus infections. In a recent report of sentinel hospital-based rotavirus surveillance from 35 nations representing each of the six WHO regions between 2001 and 2008, an average of 40% (range= 34%-45%) of hospitalizations for diarrhea among children < 5 years old were attributable to rotavirus infection. 2. Detailed recommendation: Extrapolating efficacy data from a rotavirus vaccine study performed in one population to use of same rotavirus vaccine in other populations Efficacy/effectiveness data from a rotavirus vaccine study performed in a population from one of three under-5 mortality rate categories* can be extrapolated for use in populations in the same under-5 mortality rate category. -

Rotavirus Vaccine: Questions and Answers for Health Care Providers
Rotavirus Vaccine Questions and Answers for Health Care Providers In April 2014, Manitoba Health, Seniors and Active Living launched a publicly-funded Rotavirus Immunization Program for infants born on or after March 1, 2014. In 2018, Manitoba, along with the rest of Canada, switched from RotarixTM to RotaTeq®. As of May 15, 2021, Manitoba has switched back to Rotarix®, for use in its publicly-funded Rotavirus Immunization Program, for infants born on or after April 1, 2021. This document includes an updated list of questions and answers for your reference. 1. Why is there a Rotavirus Immunization Program in Manitoba? 2. Who qualifies for publicly-funded rotavirus vaccine? 3. Which rotavirus vaccine does Manitoba use? 4. Why does the vaccine series need to be completed before eight months of age? 5. How is Rotarix® packaged? 6. How are the oral tube and cap disposed of after use? 7. Should a spit-up dose of vaccine be repeated? 8. Are there any precautions that health care providers should take when administering the oral rotavirus vaccine? 9. Oral rotavirus vaccine contains sucrose in an amount expected to have an effect on immunization injection pain. When should Rotarix® be given in relation to other vaccines to elicit a reduction in pain? 10. How do I administer Rotarix®? 11. Is additional screening for potential contraindications required prior to administering rotavirus vaccine? 12. Can infants born to mothers on immunosuppressive medication be immunized? 13. Are there any issues related to circulating maternal antibodies interfering with the response to the live attenuated vaccine? 14. Are the two rotavirus vaccines, RotaTeq® and Rotarix™, interchangeable? 15. -

Case Studies Controversies in Vaccination
European Review, Vol. 21, No. S1, S56–S61 r 2013 Academia Europæa. The online version of this article is published within an Open Access environment subject to the conditions of the Creative Commons Attribution license ,http://creativecommons.org/licenses/by/3.0/.. doi:10.1017/S1062798713000227 Session 3 – Case Studies Controversies in Vaccination ROMAN PRYMULA University Hospital Hradec Kralove, Sokolska 581, 500 05 Hradec Kralove, Czech Republic. E-mail: [email protected] Infectious diseases still jeopardize human health and even lives. In spite of the variety of advanced treatment methods, prevention is considered to be the most effective way to fight infections, and vaccination, no doubt, is one of the most effective preventive measures in the history of mankind. The vaccine controversy is based on a dispute over morality, ethics, effectiveness, and/or safety. There is no 100% safe or effective vaccine; however, benefits clearly overweigh risks. Ironically, as the numbers of cases of vaccine- preventable infectious diseases are falling, the controversies relating to vaccine safety are growing. Vaccines are generally victims of their own success. Controversies can afflict the positive acceptance of immunization, decrease the coverage and uptake and finally threaten the health of children and adults. The advent of vaccination has proven to be one of the most effective preventive measures in the history of medicine. Vaccines play a significant role in the prevention of debili- tating and, in many cases, life-threatening infectious diseases. Vaccines also provide important benefits in terms of reducing or avoiding costs associated with illness, both direct and indirect. In spite of the absence of any kind of ideal global healthcare system and lack of resources, the World Health Organisation (WHO) and the United Nations Children’s Fund (UNICEF) were able, in 1974, to establish a major global project called the Expanded Programme on Immunization (EPI). -

Pink Book Webinar Series: Rotavirus and Hepatitis a Slides
Centers for Disease Control and Prevention National Center for Immunization and Respiratory Diseases Rotavirus and Hepatitis A Pink Book Webinar Series 2018 Mark Freedman, DVM, MPH Veterinary Medical Officer Photographs and images included in this presentation are licensed solely for CDC/NCIRD online and presentation use. No rights are implied or extended for use in printing or any use by other CDC CIOs or any external audiences. Rotavirus: Disease and Vaccine Rotavirus . First identified as a cause of diarrhea in 1973 . Most common cause of severe gastroenteritis in infants and young children . Nearly universal infection by age 5 years . Responsible for up to 500,000 diarrheal deaths each year worldwide Rotavirus . Two important outer shell proteins—VP7, or G-protein, and VP4, or P-protein define the serotype of the virus . From 1996–2005, five predominate strains in the U.S. (G1–G4, G9) accounted for 90% of the isolates . G1 strain accounts for 75% of infections . Very stable and may remain viable for weeks or months if not disinfected Rotavirus Immunity . Antibody against VP7 and VP4 probably important for protection • Cell-mediated immunity probably plays a role in recovery and immunity . First infection usually does not lead to permanent immunity . Reinfection can occur at any age . Subsequent infections generally less severe Rotavirus Clinical Features . Short incubation period . First infection after 3 months of age generally most severe . May be asymptomatic or result in severe, dehydrating diarrhea with fever and vomiting . Gastrointestinal symptoms generally resolve in 3–7 days Rotavirus Complications . Infection can lead to severe diarrhea, dehydration, electrolyte imbalance, and metabolic acidosis . -

Detailed Review Paper on Rotavirus Vaccines
Rotavirus Vaccines 17 March 2009 Detailed Review Paper on Rotavirus Vaccines To be presented to the WHO Strategic Advisory Group of Experts (SAGE) on Immunization, April 2009 Ad-hoc group of experts on rotavirus vaccines Chair : G. Peter Members: T. Aguado, Z. Bhutta, L. De Oliveira, K. Neuzil, U. Parashar, D. Steele WHO Secretariat: C. Mantel, S. Wang, G. Mayers, E. Derobert Rapporteur: D. Payne 1 Rotavirus Vaccines 17 March 2009 Table of Contents I. Rotavirus Epidemiology and Rationale for Vaccination 1. Disease burden 2. Rationale for vaccination as the primary preventive measure II. Rotavirus Vaccine Efficacy and Safety in Pivotal Pre-Licensure Trials Brief summary of rotavirus vaccines 1. Rotarix ® 2. RotaTeq ® III. Newly Available Data from Clinical Trials in Africa and Asia and Post-introduction Vaccine Effectiveness Evaluations in the Americas 1. South Africa and Malawi clinical trials (Rotarix ®) 2. Hong Kong, Taiwan, and Singapore clinical trials (Rotarix ®) 3. Nicaragua post-introduction vaccine effectiveness case- control study (RotaTeq ®) 4. El Salvador post-introduction vaccine effectiveness case- control study (Rotarix ®) 5. United States post-licensure impact evaluation studies 6. Status of other ongoing studies IV. Vaccine Safety, Co-Administration, and Special Populations 1. Vaccine safety 2. Co-administration with other vaccines, particularly OPV 3. HIV-infected populations 4. Breast-feeding and Pre-term Infants V. Vaccine Schedules and Age Restrictions VI. Vaccine Cost-effectiveness and Decision-Making Regarding Program Implementation 1. Cost-effectiveness and affordability 2. Decision-making regarding vaccine introduction VII. Vaccine Program Implementation and Vaccine Delivery Logistics VIII. Integration with Diarrheal Control and Other Health Interventions and Communication 1. -
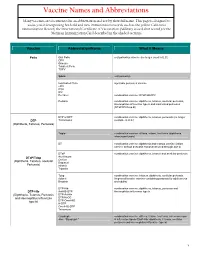
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many Vaccines Are Documented in an Abbreviation and Not by Their Full Name
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many vaccines are documented in an abbreviation and not by their full name. This page is designed to assist you in interpreting both old and new immunization records such as the yellow California Immunization Record, the International Certificate of Vaccination (Military issued shot record) or the Mexican Immunization Card described in the shaded sections. Vaccine Abbreviation/Name What it Means Polio Oral Polio oral poliovirus vaccine (no longer used in U.S.) OPV Orimune Trivalent Polio TOPV Sabin oral poliovirus Inactivated Polio injectable poliovirus vaccine eIPV IPOL IPV Pentacel combination vaccine: DTaP/Hib/IPV Pediarix combination vaccine: diphtheria, tetanus, acellular pertussis, Haemophilus influenzae type b and inactivated poliovirus (DTaP/IPV/Hep B) DTP or DPT combination vaccine: diphtheria, tetanus, pertussis (no longer DTP Tri-Immunol available in U.S.) (Diphtheria, Tetanus, Pertussis) Triple combination vaccine: difteria, tétano, tos ferina (diphtheria, tetanus pertussis) DT combination vaccine: diphtheria and tetanus vaccine (infant vaccine without pertussis component used through age 6) DTaP combination vaccine: diphtheria, tetanus and acellular pertussis DTaP/Tdap Acel-Imune Certiva (Diphtheria, Tetanus, acellular Daptacel Pertussis) Infanrix Tripedia Tdap combination vaccine: tetanus, diphtheria, acellular pertussis Adacel (improved booster vaccine containing pertussis for adolescents Boostrix and adults) DTP-Hib combination vaccine: diphtheria, tetanus, -

COVID-19 Vaccine: Critical Questions with Complicated Answers
Review Biomol Ther 29(1), 1-10 (2021) COVID-19 Vaccine: Critical Questions with Complicated Answers Mohammad Faisal Haidere1,†, Zubair Ahmed Ratan2,3,†, Senjuti Nowroz4, Sojib Bin Zaman5, You-Jung Jung6, Hassan Hosseinzadeh2,* and Jae Youl Cho7,* 1Department of Soil, Water and Environment, University of Dhaka, Dhaka 1000, Bangladesh 2School of Health & Society, University of Wollongong, NSW 2500, Australia 3Department of Biomedical Engineering, Khulna University of Engineering and Technology, Khulna 9203, Bangladesh 4Department of Chemistry, University of Dhaka, Dhaka 1000, Bangladesh 5Department of Medicine, School of Clinical Sciences, Monash University, Victoria 3800, Australia 6Biological Resources Utilization Department, National Institute of Biological Resources, Incheon 22689, Republic of Korea 7Department of Integrative Biotechnology, and Biomedical Institute for Convergence at SKKU (BICS), Sungkyunkwan University, Suwon 16419, Republic of Korea Abstract COVID-19 has caused extensive human casualties with significant economic impacts around the globe, and has imposed new challenges on health systems worldwide. Over the past decade, SARS, Ebola, and Zika also led to significant concerns among the scientific community. Interestingly, the SARS and Zika epidemics ended before vaccine development; however, the scholarly com- munity and the pharmaceutical companies responded very quickly at that time. Similarly, when the genetic sequence of SARS- CoV-2 was revealed, global vaccine companies and scientists have stepped forward to develop a vaccine, triggering a race toward vaccine development that the whole world is relying on. Similarly, an effective and safe vaccine could play a pivotal role in eradi- cating COVID-19. However, few important questions regarding SARS-CoV-2 vaccine development are explored in this review. Key Words: COVID-19, Vaccine, Vaccine backfires, Vaccine safety INTRODUCTION vember 2002 in the Guangdong province of China. -

HPV(HUMAN PAPILLOMAVIRUS)VACCINE Cervarix® W H a T Y O U N E E D T O K N O W
STATE OF CONNECTICUT DEPARTMENT OF PUBLIC HEALTH IMMUNIZATION PROGRAM PLEASE COPY THIS FOR ALL HEALTH CARE PROVIDERS IN YOUR PRACTICE TO: All Users of State Supplied Vaccine FROM: Mick Bolduc Vaccines For Children (VFC) Coordinator DATE: April 20, 2010 SUBJECT: Update on Rotavirus Vaccine The primary purpose of this communication is to provide you with an update on Rotavirus vaccine. Rotavirus Vaccine The Food and Drug Administration will be convening a meeting of its Vaccines and Related Biological Products Advisory Committee on May 7th to discuss available data concerning Glaxo Smith Kline’s Rotarix brand rotavirus vaccine as well as make recommendations for future use of the product. Since any decision concerning Rotarix will not be made until that meeting, the Immunization Program will continue to make Merck’s RotaTeq brand rotavirus vaccine available throughout the month of May. All rotavirus orders in May will be filled with RotaTeq. Providers should order RotaTeq in the space labeled “Rotavirus (Rotarix)” on the current Vaccine Order Form. When reporting your doses administered data, you can report your RotaTeq doses in the “Rotavirus” space provided on the doses administered page. As a reminder RotaTeq is administered in a 3 doses series so children who have already received one dose of Rotarix should receive 2 additional doses of RotaTeq. New Vaccine Information Statements Enclosed are the latest Vaccine Information Statements for Pneumococcal Conjugate Vaccine (updated 4/16/10) and Human Papillomavirus Vaccine (updated 3/30/10). As a reminder all Vaccine Information Statements can be downloaded at www.cdc.gov/vaccines/pubs/vis/default.htm As always, if you have any questions please contact the State Immunization Program at (860) 509-7929. -

Global Advisory Committee on Vaccine Safety (GACVS)
Global Advisory Committee on Vaccine Safety (GACVS) Report on GACVS meeting 16-17 June 2010 Peter G. Smith 1 | GACVS June 2010 report– SAGE November 2010 Safety of pandemic influenza A (H1N1) vaccines >30 pandemic (H1N1) 2009 vaccines licensed worldwide. >350 million doses of pandemic influenza vaccines administered (as of June 2010). Passive surveillance data from North America, EU, Japan and China reviewed 2 | GACVS June 2010 report– SAGE November 2010 Safety of pandemic influenza A (H1N1) vaccines – GACVS advice To date, the safety data of pandemic (H1N1) 2009 vaccines are reassuring - no unexpected safety concerns have been identified. Risk of GBS, if any, has been no more than reported previously for some seasonal trivalent inactivated influenza vaccine - the committee is waiting results of ongoing studies. So far there are no safety signals among those vaccinated during pregnancy and their offspring. Prospectively agreed upon case definitions for adverse events (e.g., anaphylaxis, GBS, convulsion) facilitate global comparisons of the safety profiles of vaccines used in different countries. 3 | GACVS June 2010 report– SAGE November 2010 Febrile seizures following seasonal influenza vaccine in Australia Increased number of reports of fever and febrile convulsions in children <5 years of age following receipt of the 2010 seasonal inactivated influenza vaccine (Fluvax) made by CSL. Seasonal influenza vaccine suspended for children <5 years of age. GACVS advice: Not aware of reports of increased fever or febrile convulsions with other 2010 seasonal vaccines. Recommends review data on the use of 2010 seasonal vaccines elsewhere. 4 | GACVS June 2010 report– SAGE November 2010 Adventitious agent in rotavirus vaccines Review of Rotarix and RotaTeq following the 25 March 2010 review of Rotarix with respect to PCV (porcine circo-virus). -

A Quick Look at Using Rotavirus (RV) Vaccines
A Quick Look at Using Rotavirus (RV) Vaccines Available Rotavirus Vaccines Vaccine Administration • Rotarix® (RV1), GlaxoSmithKline • Administer both RV vaccines orally (PO) • RotaTeq® (RV5), Merck • RV1 dosage is 1.0 mL; needs to be reconstituted, • Both RV vaccines are live, attenuated vaccines see Further Points below • RV5 dosage is 2.0 mL; is premixed • Can be given with other vaccines at the same visit • May give before or after injectable vaccines • Do not add to other liquids or food; may give Indications for Use and Schedule food/fluids before or after RV vaccine • Number of doses in series depends on RV vaccine used • Do not repeat doses that are regurgitated or spit ▪ RV1: 2-dose series at 2 months and 4 months up; count the dose and give the next dose as ▪ RV5: 3-dose series at 2, 4, and 6 months scheduled • Minimum age to start RV vaccine series is 6 weeks • Dispose vaccine vial, syringe, tube, and cap in • Maximum age for 1st dose of RV vaccine series is biologic waste container 14 weeks, 6 days • Maximum age for last dose of RV vaccine series is 8 months, 0 days Storage and Handling • Minimum interval between RV vaccine doses is 4 weeks • Store vaccine in the refrigerator at 36ºF to 46ºF • If both RV vaccines are available, continue series with the (2ºC to 8ºC) same product (RV1 or RV5); if not, use the product in • Do not freeze stock • Pharmaceutical-grade (purpose-built) units are • If any dose in RV vaccine series is RV5 or the brand is preferred for vaccine storage unknown, give the 3-dose series • Keep in the original boxes and protect from light CONTRAINDICATIONS • Severe allergic reaction (e.g., anaphylaxis) after a previous dose of vaccine or to a vaccine component ▪ Note: Latex rubber is contained in the RV1 oral applicator. -

Vchip / Champ / Vdh Covid-19 Updates
VCHIP / CHAMP / VDH COVID-19 UPDATES Wendy Davis, MD FAAP - Vermont Child Health Improvement Program, UVM Breena Holmes, MD FAAP – Director of Maternal & Child Health, Vermont Department of Health June 29, 2020 Technology Notes 1) All participants will be muted upon joining the call. If you dialed in or out, unmute by pressing #6 to ask a question (and press *6 to mute). Presenters: Please avoid the use of speakerphone and make sure your computer speaker is muted if you dialed in via phone. 2) To ask or respond to a question using the Chat box, type your question and click the icon or press Enter to send. June 29, 2020 2 Overview Launch of the “Tipping Your Cap” campaign by Major League Baseball (#TipYourCap2020) June 25, 1978: inaugural unfurling of the Rainbow Flag (at S. F. Pride) Today is also Please Take My Children to Work Day This week: REVISED call schedule Mon & Wed ONLY Situation and AAP updates Today’s Media Briefing Practice Issue: Update on COVID-19 Outbreaks, Serology, & Vaccines Drs. Bill Raszka and Ben Lee, Pediatric Infectious Diseases, UVM Children’s Hosp. Q & A, Discussion [Please note: the COVID-19 situation continues to evolve very rapidly – so the information we’re providing today may change quickly] June 29, 2020 3 Situation update Weekend (new) cases not associated with outbreaks. Fairhaven: significant community testing over weekend with no positives. https://www.healthvermont.gov/response/coronavirus-covid-19/current-activity-vermont#dashboard June 29, 2020 4 Situation update https://www.healthvermont.gov/response/coronavirus-covid-19/current-activity-vermont#dashboard