The EL34 Power Tube HI-'I
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

MI Amplification
MI Amplification Owner’s Manual 1 1. Welcome ....................................................................................................................................... 4 2. Precautions................................................................................................................................... 5 3. Amp Overview .............................................................................................................................. 6 3.1. Preamp.............................................................................................................................................. 6 3.2. Power Amp ....................................................................................................................................... 6 3.3. FX Loops .......................................................................................................................................... 7 3.4. Operating Modes ............................................................................................................................. 7 4. Getting Started ............................................................................................................................. 8 5. The Channels ............................................................................................................................... 9 5.1. Introduction ..................................................................................................................................... 9 5.2. Channel 1 ......................................................................................................................................... -

Ronnie Earl for a Blackface Vibroverb Today? Surely Something North of $4,000
Mountainview Publishing, LLC INSIDE the The Truth… Rarely given, we know it when we hear it, and you The Player’s Guide to Ultimate Tone TM can only get it here… $15.00 US, December 2013/Vol.15 NO.2 Report The Kansas Tornado… Another immensely tone- Truth ful classic amp that went unclaimed “Three chords and the truth – that’s what a country song is.” – Willie Nelson Size matters… Pressure is being asked point-blank which guitar, amplifier or pickup to buy. This happens frequent- The Warehouse ly enough that we have learned to pose probing personal questions in search of an answer… Well, G15A Alnico what do you think you might want and why? We don’t always know what we want until we want it, Big Fifteen and we still couldn’t always tell you why. Sometimes the things that last seem to materialize out of no where with little forethought or insight, as if we were meant to have them, and that’s the truth. 4 Richard Goodsell and the evolution of the Goodsell Super 17 Our review of the Super 17 Mark IV 10 The Goodsell overdrive 10 John McGuire Guitars Our interview with John McGuire & review of the McGuire Tradition 14 The Eastwood Jupiter Pro… We also must confess that we don’t always understand what motivates guitar players when it Simply cool comes to choosing amplifiers today. This is nothing new – most of the amps we own are immensely in every way toneful classic keepers that were ignored by potential buyers due to stripped or recovered original cabinets or a replaced transformer. -

Valve Biasing
VALVE AMP BIASING Biased information How have valve amps survived over 30 years of change? Derek Rocco explains why they are still a vital ingredient in music making, and talks you through the mysteries of biasing N THE LAST DECADE WE HAVE a signal to the grid it causes a water as an electrical current, you alter the negative grid voltage by seen huge advances in current to flow from the cathode to will never be confused again. When replacing the resistor I technology which have the plate. The grid is also known as your tap is turned off you get no to gain the current draw required. profoundly changed the way we the control grid, as by varying the water flowing through. With your Cathode bias amplifiers have work. Despite the rise in voltage on the grid you can control amp if you have too much negative become very sought after. They solid-state and digital modelling how much current is passed from voltage on the grid you will stop have a sweet organic sound that technology, virtually every high- the cathode to the plate. This is the electrical current from flowing. has a rich harmonic sustain and profile guitarist and even recording known as the grid bias of your amp This is known as they produce a powerful studios still rely on good ol’ – the correct bias level is vital to the ’over-biased’ soundstage. Examples of these fashioned valves. operation and tone of the amplifier. and the amp are most of the original 1950’s By varying the negative grid will produce Fender tweed amps such as the What is a valve? bias the technician can correctly an unbearable Deluxe and, of course, the Hopefully, a brief explanation will set up your amp for maximum distortion at all legendary Vox AC30. -
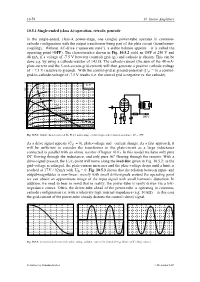
Operation, Tetrode, Pentode in the Single-Ended, Class-A
10-76 10. Guitar Amplifiers 10.5.1 Single-ended (class A)-operation, tetrode, pentode In the single-ended, class-A power-stage, one (single) power-tube operates in common- cathode configuration with the output transformer being part of the plate circuit (transformer- coupling). Without AC-drive (“quiescent state”), a stable balance appears – it is called the operating point (OPP). The characteristics shown in Fig. 10.5.2 yield an OPP at 250 V and 48 mA, if a voltage of -7.5 V between (control) grid (g1) and cathode is chosen. This can be done e.g. by using a cathode-resistor of 142 Ω. The cathode-current (the sum of the 48-mA- plate-current and the 5-mA-screen-grid-current) will then generate a positive cathode-voltage of + 7.5 V (relative to ground). With the control-grid at ground-potential (Ug1 = 0) a control- grid-to-cathode-voltage of -7.5 V results (i.e. the control grid is negative vs. the cathode). Fig. 10.5.2: Output characteristics of the EL84, power-stage circuit (single-ended class-A operation). AP = OPP As a drive signal appears (Ug1 ≠ 0), plate-voltage and –current change. As a first approach, it will be sufficient to consider the transformer in the plate-circuit as a large inductance connected in parallel with an ohmic resistor (Chapter 10.6). In this model we have only pure DC flowing through the inductance, and only pure AC flowing through the resistor. With a drive-signal present, the Ua/Ia-point will move along the load-line given in Fig. -

VACUUM TUBE VALLEY Fall 1995 Price $6.50
Pub/ish«lQuarterly Celebrating the History and QlIOlity of Vocllum Tube Te<lmology luue 2 Vo/LlI7U! I VACUUM TUBE VALLEY Fall 1995 Price $6.50 Magnum SE Amplifier Da\-c Wolze rec.:nrlydesigned and built an SE amp with power and punch. Page 17 ...... Tube Review: EL·34 In one of existence since 1953 and th(Omost popular audio tubes of all time, rhe EL-34 has many variarions and performance characteristics. Page 8 Heath W-6M Heathkit: Early Tube Hi-Fi years. In This Issue .. manufacturer of Heathkit was the largest d�c Ironic kits in the US, alone time, selling over ntbe bldustry News 350 different types of kts. Learn more aboUl Check OUt the latest happen gs i in in the the early days of Heath Hi-Fi. Page 3 world of vacuum tubes. Learn the results of a recent survey of tuhe dislfih mors and sellers conducted by VIV. MU/UlrdEL-34s Harbouroudines the latest Ilem and Eric Early Cinema Sound Views. Page 15 vrv examines an early \xre�ternElectric [heater sound system. Page 24 Guitar Amplifiers Learn about how to get the best guitar tone. Chaclie Kittleson interviews Terry Buddingh, Tube Amp Expert from GuiTdrPi4yer Magazine. Page 20 Heatb w-4AM Tube Matching: Get the best soutuisfrom your amp. Matched tubes arc essential for opti mum performance from push-pull amps. n John Atwood explai s tube matching techniques for the layman. Page 22 Vacuum Tube Valleyis published quarterly for electronic enthusiasts interested in the See (Jur newfiatures in this months colorfv1 past, present and fvture af yocuum tube electronics. -

Push - Pull Audiophile - Output (1608-1650 "A" Series)
Push - Pull Audiophile - Output (1608-1650 "A" Series) PUSH - PULL "EASY HOOK-UP" "CLASSIC" TUBE TYPE - ULTRA-LINEAR OUTPUT TRANSFORMERS • NEW & Improved version of our 1608-1650 series output transformers (re-designed secondaries for easy hook- up of secondary loads) • Designed for push-pull tube output circuits. o • Enclosed (shielded), 4 slot, above chassis Type "X" mounting. • Frequency response 30 Hz. to 30 Khz. at full rated power (+/- 1 db max. - ref. 1 Khz) minimum. Except the Audi 1650E (70 Hz. to 30 Khz. +/- 1 db max. - ref. 1 Khz.) be • Insulated flexible leads 8" min. • All units (except the 1650G) include 40% screen taps for Ultra-Linear operation (if desired). Tu • Typical applications - Push-Pull: triode, Ultra-Linear pentode, and tetrode connected audio output. The 1650G does NOT have primary screen taps and will not support "Ultra-Linear" applications. Audio Dimensions (Inches) Part Primary Max. DC Secondary Wt. Watts E G Number Impedance Per Side Impedance A B C D Lbs. (RMS) +/- 1/16” Slot 1608A 10 8,000 C.T. 100 ma. 4-8-16 2.50 2.75 3.06 2.00 1.69 .203 x .38 2.5 1609A 10 10,000 C.T. 100 ma. 4-8-16 2.50 2.75 3.06 2.00 1.69 .203 x .38 2.5 1615A 15 5,000 C.T. 100 ma. 4-8-16 2.50 3.25 3.06 2.00 2.19 .203 x .38 3.25 1650E 15 8,000 C.T. 100 ma. 4-8-16 2.50 3.25 3.06 2.00 2.50 .203 x .38 3.5 1620A 20 6,600 C.T. -

Bias-Easy 1200™ Bias Tester Instructions
Bias-Easy 1200™ Bias Tester Instructions 1. Turn off your amp, and allow to cool down. The Bias-Easy 1200™ is primarily for amplifiers using four 8-pin power tubes with a bias adjustment potentiometer available inside or on the chassis underside or back panel of the amp. 2. Remove one of the power tubes (6L6, EL34, 5881, 6550, 6V6, KT66, KT77, etc) 3. Insert a Bias-Easy™ probe into the power tube socket, making sure to line up the key on the probe post with the key in the center hole of the socket. Repeat for the second, third and fourth power tubes with each of the remaining probes. If your amp only has two power tubes, we suggest using the A and B probes. The C and D probes may be unplugged from the Bias-Easy™ in that case. 4. Insert each tube you removed into each socket of the Bias-Easy™. Make sure the key on the tube base is properly lined up with the key on the center hole of the socket. All power tube(s) must be in place in their sockets during testing. 5. Make certain that a speaker is connected to the amp. If this is an amp head, connect a cable between the speaker jack and the speaker cabinet. 6. Make sure all bias probes to be used in the testing are connected to the jacks at the top of the Bias-Easy™. Connect the power supply cord to the jack on the bottom of the Bias-Easy™, and connect the USB connector on the cord to the power supply. -

Využití Elektronek V Současné Době
Jihočeská univerzita v Českých Budějovicích Pedagogická fakulta Katedra aplikované fyziky a techniky Diplomová práce Využití elektronek v současné době Vedoucí práce: Ing. Michal Šerý, Ph.D. Autor práce: Bc. Josef Čepička Studijní obor: Učitelství fyziky a výpočetní techniky s elektronikou pro 2. stupeň ZŠ, kombinované studium České Budějovice 2013 Anotace Cílem této diplomové práce je zkonstruování ukázkového modelu elektronkového zesilovače s možností měření procházejícího signálu ve více bodech a možností náhledu do zapojení a konstrukce zesilovače. Na začátku práce je popsáno několik zásadních témat týkajících se elektronek a zapojení s nimi, jako například princip zesílení signálu pomocí elektronek, třídy nízkofrekvenčních zesilovačů a popis několika typů nežádoucích zkreslení. Další část práce pojednává o samotném návrhu zkonstruovaného zesilovače, popisu vzniku a osazení desek plošných spojů a použitých elektronkách a transformátorech. Následující část práce je věnována měření základních vlastností zesilovače a měření vlastností elektronek pomocí přístroje TESLA BM215A. V závěrečné kapitole jsou uvedeny technické parametry zesilovače a fotodokumentace. Abstract Aim of this thesis is to construct a demonstration model of a vacuum tube amplifier with a possibility of measurement of signal on more places and a possibility to see the wiring and construction of the amplifier. The first part of the work treats some important themes concerning vacuum tubes and wiring with them, for example a principle of signal intensification by vacuum tubes, categories of low-frequency amplifiers and description of several types of undesirable distortions. Next part deals with the design of the constructed amplifier, the description of the construction itself and placement of the components on the printed circuit boards and used vacuum tubes and transformers. -

Tube Output (10 - 280 Watts) (1608-1650 Series) - Hammond Mfg
9/6/2021 Tube Output (10 - 280 Watts) (1608-1650 Series) - Hammond Mfg. Quality Products. Service Excellence. Tube Output (10 - 280 Watts) 1608-1650 Series Push-Pull - HI-FI Features Please see our NEW & improved versions (easy wire secondary series). Designed for push-pull tube output circuits. Enclosed (shielded), 4 slot, above chassis Type "X" mounting. Frequency response 30 Hz. to 30 Khz. at full rated power (+/- 1 db max. - ref. 1 Khz) minimum. Insulated flexible leads 8" min. Manufactured with plastic coil forms for coil support and insulation. Typical applications - Push-Pull: triode, Ultra-Linear pentode, pentode and tetrode connected audio output. Due to the unique interleaving of the windings BOTH secondary windings must be engaged to meet specifications (see hook-up diagrams below). For the "ultimate" in Push-Pull output see our line of epoxy potted output transformers. 1645 Only Secondary Connections (Due to the unique interleaving of the windings BOTH secondary windings must be engaged to meet specifications) To hook up 4/8/16 ohm secondary loads - see schematic (do not use the white wire). To hook up secondary to 70V loads, jumper Blk/Yel wire to Grn wire. Connect load to Blk and White wires. Gallery Audio Primary Maximum Secondary Dimensions Watts Impedance DC Impedance E G Weight Part No. (RMS) (Ohms) Per Side (Ohms) A B C D +/- 1/16" Slot (lbs.) https://www.hammfg.com/electronics/transformers/classic/1608-1650 1/2 9/6/2021 Tube Output (10 - 280 Watts) (1608-1650 Series) - Hammond Mfg. Audio Primary Maximum Secondary Dimensions Watts Impedance DC Impedance E G Weight Part No. -

Tube Pinouts
bustedgear.com VACUUM TUBE PINOUT SHEETS INDEX Tube Page Tube Page Tube Page Tube Page 5AR4 5 6FQ7 30 12DW7 22 7025 22 5AU4 6 6FV8A 28 12FX5 12 7027A 18 5BR8 28 6J7 13 12SL7 16 7189 25 5BS8 23 6K11 35 12SN7 16 7189A 25 5FV8 28 6L6 10 7199 29 5U4 6 6N7 15 18GD6A 11 7591A 19 5V3 6 6Q11 35 35Z5 7 76 3 5Y3 6 6SC7 21 36AM3 4 7868 33 5Z3 2 6SJ7 20 50BM8 27 6SL7 16 50L6 10 ECC81 22 6AU6 11 6SN7 16 5751 22 ECC82 22 6BM8 27 6V4 32 5881 10 ECC83 22 6BQ5 25 6V6 10 6072A 22 ECC832 22 6BR8A 28 60FX5 12 ECC88 23 6C4 8 12AT7 22 6201 22 ECL82 27 6C6 9 12AU6 11 6550 14 EL34 17 6C10 34 12AU7 22 6679 22 EL84 25 6CA4 32 12AX7 22 6680 22 EZ80 32 6CA7 17 12AY7 22 6681 22 EZ81 32 6CG7 30 12BH7 22 6922 23 GZ34 5 6CX8 26 12BV7 24 KT66 10 6DJ8 23 12BY7A 24 KT88 17 6EU7 31 12DQ7 24 UCL82 27 5Z3 PIN DIAGRAM: 4C Page 2 WIRING SIDE OF SOCKET IS SHOWN INDEX AMPLIFIER MAKE: MODEL: FULL-WAVE RECTIFIER SERIAL NO: VOLTAGE READINGS PIN ELECTRODE a: b: c: d: e: f: 1 Filament 2 d2 - Plate 3 d1 - Plate 4 Filament TERMINAL ABBREVIATIONS: H = Heater End (Unpolarized). HM = Heater Tap. IC = Do Not Use (Internal Connection). IS = Internal Shield (Electrostatic). K = Cathode. NC = No Internal Connection. S = Metal Shell. SUBSCRIPTS FOR MULTI-UNIT TUBES: b = Beam Power Unit. -

Yellow Jackets Dimensions Sheet
TUBE CONVERTERS Yellow Jackets® tube converters allow EL84 power tubes to be used in place of the most common guitar amp power tubes including 6L6, EL34, 6V6, 7027, 6550 and 7591. Most Yellow Jackets® screen No provide a substantial output power grid Connection reduction and a “self-bias” Class A 9 1 No EL84 configuration for the EL84 so that no Connection control bias adjustment is required. Yellow 8 2 grid Jackets® are like getting a whole new amp. plate 7 3 cathode + suppressor grid Yellow Jackets® Types No 6 4 Connection 5 filament YJS p. 2 filament YJSHORT p. 3 YJC p. 3 YJ20 p. 4 YJUNI p. 4 1 9 YJ7591 p. 4 YJR p. 5 1 8 screen control grid 4 5 grid plate 3 6 No Connection 2 7 filament filament No 1 8 cathode Connection + suppressor grid 1 Why would I want to convert to EL84's using Yellow Jackets®? Every power tube type offers a different characteristic sound and feel. EL84's have a very tight and focused sound which has become world renown by their use in the British VOX™ AC30 guitar amplifiers. Additionally, most Yellow Jackets® converters will produce a substantial maximum power reduction (50% to 90%) making it easier to find that sweet, warm mix of preamp and power amp distortion at a lower volume. Yellow Jackets also convert the power tube bias to “self-bias” Class A so that no bias adjustment is necessary. You can switch back and forth between EL84's and your amplifier’s original power tubes without rebiasing. -

Getter Pumping
Getter pumping C. Benvenuti R&B Energy Research, Geneva, Switzerland Abstract A surface may provide a useful pumping action when able to retain adsorbed gas molecules for the duration of a given experiment. To fulfil this condition at room temperature, strong binding forces, as those resulting from chemical reactions, are required. Materials able to react with gases to form stable chemical compounds are called getters. The two main families of getters (evaporable and non-evaporable, or NEG) are presented and discussed. Special emphasis is placed on the NEG strips currently used for the vacuum systems of particle accelerators, and on the newly developed NEG thin-film coatings, in view of their possible future applications. 1 Generalities 1.1 Molecular mean free path λ 7.3T λ = cm 20π 2 10 rp0 π 2 where T is the absolute temperature (K), p the pressure (Torr) and r0 the collision cross-section 2 π 215= − 2 λ = 3 –3 6 (cm ). For N2, r0 4.26 × 10 cm . At 295 K, 5 × 10 p (i.e. 5 cm at 10 Torr, or 5 × 10 cm at 10–9 Torr). Only molecular flow ( λ >> wall distance) will be considered hereafter. 1.2 Conductance and surface pumping The conductance C of an orifice (zero wall thickness approximation) may be expressed as CTM= 3.64()12A s−−12 cm with T = absolute temperature (K), M = molecular weight. If all molecules impinging on a surface are captured, C represents the specific pumping speed S of the surface. More generally STM= α 3.64()12A s−−12 cm where α is the sticking probability ()01≤≤α .