DNA Before Proteins? Recent Discoveries in Nucleic Acid Catalysis Strengthen the Case
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(12) Patent Application Publication (10) Pub. No.: US 2013/0203610 A1 Meller Et Al
US 20130203610A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2013/0203610 A1 Meller et al. (43) Pub. Date: Aug. 8, 2013 (54) TOOLS AND METHOD FOR NANOPORES Related U.S. Application Data UNZIPPING-DEPENDENT NUCLECACID (60) Provisional application No. 61/318,872, filed on Mar. SEQUENCING 30, 2010. (75) Inventors: Amit Meller, Brookline, MA (US); Alon Publication Classification Singer, Brighton, MA (US) (51) Int. Cl. (73) Assignee: TRUSTEES OF BOSTON CI2O I/68 (2006.01) UNIVERSITY, Boston, MA (US) (52) U.S. Cl. CPC .................................... CI2O I/6874 (2013.01) (21) Appl. No.: 13/638,455 USPC ................................................. 506/6:506/16 (57) ABSTRACT (22) PCT Filed: Mar. 30, 2011 Provided herein is a library that comprises a plurality of molecular beacons (MBs), each MB having a detectable (86). PCT No.: PCT/US2O11AO3O430 label, a detectable label blocker and a modifier group. The S371 (c)(1), library is used in conjunction with nanopore unzipping-de (2), (4) Date: Apr. 17, 2013 pendent sequencing of nucleic acids. Patent Application Publication Aug. 8, 2013 Sheet 1 of 18 US 2013/020361.0 A1 . N s Patent Application Publication Aug. 8, 2013 Sheet 2 of 18 US 2013/020361.0 A1 I’9IAI ::::::::::: Dº3.modoueN Patent Application Publication Aug. 8, 2013 Sheet 3 of 18 US 2013/020361.0 A1 Z’9IAI ~~~~~~~~~);........ Patent Application Publication Aug. 8, 2013 Sheet 4 of 18 US 2013/020361.0 A1 s :·.{-zzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzzz iii. 9 iii.338 lii &S Patent Application Publication Aug. 8, 2013 Sheet 5 of 18 US 2013/020361.0 A1 s r Patent Application Publication Aug. -

Alternative Biochemistries for Alien Life: Basic Concepts and Requirements for the Design of a Robust Biocontainment System in Genetic Isolation
G C A T T A C G G C A T genes Review Alternative Biochemistries for Alien Life: Basic Concepts and Requirements for the Design of a Robust Biocontainment System in Genetic Isolation Christian Diwo 1 and Nediljko Budisa 1,2,* 1 Institut für Chemie, Technische Universität Berlin Müller-Breslau-Straße 10, 10623 Berlin, Germany; [email protected] 2 Department of Chemistry, University of Manitoba, 144 Dysart Rd, 360 Parker Building, Winnipeg, MB R3T 2N2, Canada * Correspondence: [email protected] or [email protected]; Tel.: +49-30-314-28821 or +1-204-474-9178 Received: 27 November 2018; Accepted: 21 December 2018; Published: 28 December 2018 Abstract: The universal genetic code, which is the foundation of cellular organization for almost all organisms, has fostered the exchange of genetic information from very different paths of evolution. The result of this communication network of potentially beneficial traits can be observed as modern biodiversity. Today, the genetic modification techniques of synthetic biology allow for the design of specialized organisms and their employment as tools, creating an artificial biodiversity based on the same universal genetic code. As there is no natural barrier towards the proliferation of genetic information which confers an advantage for a certain species, the naturally evolved genetic pool could be irreversibly altered if modified genetic information is exchanged. We argue that an alien genetic code which is incompatible with nature is likely to assure the inhibition of all mechanisms of genetic information transfer in an open environment. The two conceivable routes to synthetic life are either de novo cellular design or the successive alienation of a complex biological organism through laboratory evolution. -

Threose Nucleic Acid
2/23/11! Something else…! •" Neither the chicken nor the egg came first! Alternative Ideas! •" Transitional forms that were later discarded! Or was it the “egkin”?! Some experiments with peptide nucleic acid! Threose Nucleic Acid (TNA)! (PNA).! PNA: Peptide backbone with bases! •" Threose is one of two sugars with a four- sided ring! Can act as template for polymerization of RNA! •" Fewer issues with incorrect linkages, From activated nucleotides! selection of correct handedness! (Böhler, et al., Nature, 376, 578! •" Replace ribose sugar in RNA with threose! & comments by Piccirilli, pg. 548 17 Aug. 1995! •" Can base pair with RNA! •" Could have preceded RNA! PNA could be simpler to form under prebiotic conditions! Main point is that a simpler thing (not necessarily PNA) ! could have preceded RNA! 1! 2/23/11! Focus on Energy! Membranes! G. Wächtershäuser! Inorganic - organic connection! •" Membranes provide enclosure! FeS2 ! (Iron pyrite)! –"Also fundamental for metabolism! Attracts negatively charged molecules ! •" Membranes never arise from scratch! Surface catalysis provides energy via formation from! –"Always passed down and added to! FeS + H2S! –"All derived from ancestral cell! •" T. Cavalier-Smith proposes membranes! Scene is hot sulfur vents on sea floor! –"Plus nucleic acid formed “ob-cell”! Some successes in simulations! Amino acids formed peptide bonds! –"Merger of 2 ob-cells formed first cell! Thioester World! H O 1." Need precursor to RNA world! H H S H O Thioester! S C. de Duve! C C O 2. !Need energy conversion! In Vital Dust! O !Protometabolism! Thiol + Carboxyl! Background:! H O H H O H O Ester! O Thiols involved in metabolism, particularly in ancient ! C C pathways! O O Hydroxyl + Carboxyl! Also can catalyze ester formation by group transfer! Reactions! e.g. -

Darwinian Evolution of an Alternative Genetic System Provides Support for TNA As an RNA Progenitor Hanyang Yu†,Suzhang† and John C
ARTICLES PUBLISHED ONLINE: 10 JANUARY 2012 | DOI: 10.1038/NCHEM.1241 Darwinian evolution of an alternative genetic system provides support for TNA as an RNA progenitor Hanyang Yu†,SuZhang† and John C. Chaput* The pre-RNA world hypothesis postulates that RNA was preceded in the evolution of life by a simpler genetic material, but it is not known if such systems can fold into structures capable of eliciting a desired function. Presumably, whatever chemistry gave rise to RNA would have produced other RNA analogues, some of which may have preceded or competed directly with RNA. Threose nucleic acid (TNA), a potentially natural derivative of RNA, has received considerable interest as a possible RNA progenitor due to its chemical simplicity and ability to exchange genetic information with itself and RNA. Here, we have applied Darwinian evolution methods to evolve, in vitro, a TNA receptor that binds to an arbitrary target with high affinity and specificity. This demonstration shows that TNA has the ability to fold into tertiary structures with sophisticated chemical functions, which provides evidence that TNA could have served as an ancestral genetic system during an early stage of life. he question of why nature chose ribofuranosyl nucleic acids as like DNA and RNA, can fold into structures with complicated func- the molecular basis of life’s genetic material has prompted a tions—a prerequisite for any RNA progenitor in a hypothetical Tsystematic analysis of nucleic acid structure with regard to pre-RNA world. the chemical etiology of RNA1. Results from these studies reveal that several potentially natural RNA alternatives are capable of Results and discussion Watson–Crick base pairing, demonstrating the capacity for these The discovery by Eschenmoser and colleagues that TNA can molecules to store genetic information. -

12.12.2017 1 CHAPTER 1 OLIGONUCLEOTIDES Part 2
12.12.2017 Canonical nucleobase pairing and alternative base pairs CHAPTER 1 OLIGONUCLEOTIDES Part 2 – noncanonical nucleobases Natural and non-natural base pairs that function in polymerase reactions Self-assembly of whole genes and DNA nanostructures The technology tested by assembly of the kanamycin-resistance gene and growing the bacteria in the environment containing kanamycin after assembly and conversion of that gene. S. Benner et al., Beilstein J. Org. Chem. 2014, 10, 2348–2360. doi:10.3762/bjoc.10.245 1 12.12.2017 AEGIS – Artificially Expanded Genetic Information System AEGIS – Artificially Expanded Genetic Information System ZP CG S. Benner et al., Beilstein J. Org. Chem. 2014, 10, 2348–2360. doi:10.3762/bjoc.10.245 S. Benner et al., J. Am. Chem. Soc., 2011 , 133 (38), pp 15105–15112 AEGIS – Permanent orthogonal nucleobases surviving PCR ACGTZP-DNA crystal structures Electron density presented to the minor groove recognition site by polymerases „ minor groove scanning hypothesis” Error rate 0,2% per a PCR cycle – both removal and incorporation of Z and P the artificial genetic system capable to evolve. Pol: Deep Vent – 2 Z/P, Taq/Phu – 3-4 Z/P 18-mers: 2+2 Z:P pairs B-DNA dZTP ( deprotonated ) at higher pH pairs slightly with G 6 consecutive Z:P A-DNA loss of some Z, but also gain of some new Z mutants. 0,1 nm wider, but otherwise alike G:C pairs S. Benner et al., J. Am. Chem. Soc., 2011 , 133 (38), pp 15105–15112 S. Benner et al., J. Am. Chem. Soc., 2015, 137, pp 6947–6955 2 12.12.2017 Unnatural aminoacid incorporation using a noncanonical base pair (A) The coupled transcription–translation system using the nonstandard codon– anticodon interaction for the site-specific incorporation of 3-chlorotyrosine into the Ras protein. -

Evaluating TNA Stability Under Simulated Physiological Conditions
Bioorganic & Medicinal Chemistry Letters 26 (2016) 2418–2421 Contents lists available at ScienceDirect Bioorganic & Medicinal Chemistry Letters journal homepage: www.elsevier.com/locate/bmcl Evaluating TNA stability under simulated physiological conditions Michelle C. Culbertson a, Kartik W. Temburnikar a, Sujay P. Sau a,c, Jen-Yu Liao a,c, Saikat Bala a,c, ⇑ John C. Chaput a,b,c, a Biodesign Institute, Arizona State University, Tempe, AZ 85287-5301, USA b Department of Chemistry and Biochemistry, Arizona State University, Tempe, AZ 85287-5301, USA c Department of Pharmaceutical Sciences, University of California, Irvine, CA 92697-3958, USA article info abstract Article history: Chemically modified oligonucleotides are routinely used as diagnostic and therapeutic agents due to their Received 12 March 2016 enhanced biological stability relative to natural DNA and RNA. Here, we examine the biological stability Revised 30 March 2016 of a-L-threofuranosyl nucleic acid (TNA), an artificial genetic polymer composed of repeating units of Accepted 31 March 2016 0 0 a-L-threofuranosyl sugars linked by 2 ,3 -phosphodiester bonds. We show that TNA remains undigested Available online 1 April 2016 after 7 days of incubation in the presence of either 50% human serum or human liver microsomes and is stable against snake venom phosphordiesterase (a highly active 30 exonuclease). We further show that Keywords: TNA will protect internal DNA residues from nuclease digestion and shield complementary RNA strands Threose nucleic acid from RNA degrading enzymes. Together, these results demonstrate that TNA is an RNA analogue with Biological stability RNA analogue high biological stability. Ó 2016 Elsevier Ltd. All rights reserved. -

Theranostics Molecular Beacons of Xeno-Nucleic Acid for Detecting
Theranostics 2013, Vol. 3, Issue 6 395 Ivyspring International Publisher Theranostics 2013; 3(6):395-408. doi: 10.7150/thno.5935 Review Molecular Beacons of Xeno-Nucleic Acid for Detecting Nucleic Acid Qi Wang2, Lei Chen1, Yitao Long1, He Tian1 and Junchen Wu1, 1. Key Lab for Advanced Materials and Institute of Fine Chemicals, East China University of Science and Technology, China. 2. College of Public Health, Nantong University, China. Corresponding author: Junchen Wu, Meilong 130, Shanghai 200237, China. Telephone: (+86) 21-6425-3674, fax: (+86) 21-6425-2258, E-mail: [email protected]. © Ivyspring International Publisher. This is an open-access article distributed under the terms of the Creative Commons License (http://creativecommons.org/ licenses/by-nc-nd/3.0/). Reproduction is permitted for personal, noncommercial use, provided that the article is in whole, unmodified, and properly cited. Received: 2013.01.23; Accepted: 2013.04.10; Published: 2013.05.05 Abstract Molecular beacons (MBs) of DNA and RNA have aroused increasing interest because they allow a continuous readout, excellent spatial and temporal resolution to observe in real time. This kind of dual-labeled oligonucleotide probes can differentiate between bound and unbound DNA/RNA in homogenous hybridization with a high signal-to-background ratio in living cells. This review briefly summarizes the different unnatural sugar backbones of oligonucleotides combined with fluoro- phores that have been employed to sense DNA/RNA. With different probes, we epitomize the fundamental understanding of driving forces and these recognition processes. Moreover, we will introduce a few novel and attractive emerging applications and discuss their advantages and dis- advantages. -

Modeling Prebiotic Catalysis with Nucleic Acid-Like Polymers and Its Implications for the Proposed RNA World*
Pure Appl. Chem., Vol. 76, No. 12, pp. 2085–2099, 2004. © 2004 IUPAC Modeling prebiotic catalysis with nucleic acid-like polymers and its implications for the proposed RNA world* S. G. Srivatsan‡ Department of Chemistry, Indian Institute of Technology-Kanpur, Kanpur-208016 (UP), India Abstract: The theory that RNA molecules played a pivotal role in the early evolution of life is now widely accepted. Studies related to this hypothetical “RNA world” include three major areas: the formation of precursors for the first RNA molecules, the polymerization process, and the potential of RNA to catalyze chemical and biochemical reactions. Several chemical and biochemical studies performed under simulated prebiotic conditions support the role of RNA as both genetic as well as catalytic material. However, owing to the lack of credible mechanism for de novo nucleic acid synthesis and the hydrolytic instability of RNA mole- cules, there has been some serious discussion of whether biopolymers that closely resembled nucleic acid preceded the “RNA world”. In this context, an overview of prebiotic chemistry, the role of mineral surface, and the significance of studies related to RNA-like polymers in the origin of life are presented here. INTRODUCTION Though life that exists today and in the geological record is based on DNA genomes and protein en- zymes, there is convincing evidence that hypothesizes the origin of life based on RNA [1]. This hypo- thetical primordial era is referred to as the “RNA world” [2]. The “RNA world” hypothesis assumes that all modern organisms are descendants of a prebiotic self-replicating moiety, which utilized RNA as both genetic as well as catalytic material. -
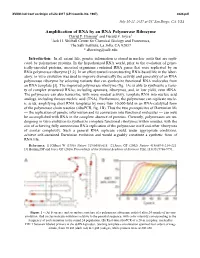
Amplification of RNA by an RNA Polymerase Ribozyme David P
XVIIIth Intl Conf on Origin of Life 2017 (LPI Contrib. No. 1967) 4024.pdf July 16-21, 2017 at UC San Diego, CA, USA Amplification of RNA by an RNA Polymerase Ribozyme David P. Horning1 and Gerald F. Joyce1 1Jack H. Skirball Center for Chemical Biology and Proteomics, The Salk Institute, La Jolla, CA 92037 * [email protected] Introduction: In all extant life, genetic information is stored in nucleic acids that are repli- cated by polymerase proteins. In the hypothesized RNA world, prior to the evolution of genet- ically-encoded proteins, ancestral organisms contained RNA genes that were replicated by an RNA polymerase ribozyme [1,2]. In an effort toward reconstructing RNA-based life in the labor- atory, in vitro evolution was used to improve dramatically the activity and generality of an RNA polymerase ribozyme by selecting variants that can synthesize functional RNA molecules from an RNA template [3]. The improved polymerase ribozyme (fig. 1A) is able to synthesize a varie- ty of complex structured RNAs, including aptamers, ribozymes, and, in low yield, even tRNA. The polymerase can also transcribe, with more modest activity, template RNA into nucleic acid analogs, including threose nucleic acid (TNA). Furthermore, the polymerase can replicate nucle- ic acids, amplifying short RNA templates by more than 10,000-fold in an RNA-catalyzed form of the polymerase chain reaction (riboPCR, fig. 1B). Thus the two prerequisites of Darwinian life — the replication of genetic information and its conversion into functional molecules — can now be accomplished with RNA in the complete absence of proteins. Currently, polymerases are un- dergoing in vitro evolution to synthesize complete functional ribozymes within minutes, with the aim of achieving fully autonomous RNA replication of the polymerase itself and other ribozymes of similar complexity. -

Evolution of Functional Nucleic Acids in the Presence of Nonheritable Backbone Heterogeneity
Evolution of functional nucleic acids in the presence of nonheritable backbone heterogeneity Simon G. Trevinoa, Na Zhanga, Mark P. Elenkoa, Andrej Luptákb, and Jack W. Szostaka,1 aHoward Hughes Medical Institute, Center for Computational and Integrative Biology, and Department of Molecular Biology, Simches Research Center, Massachusetts General Hospital, Boston, MA 02144; and bDepartments of Pharmaceutical Sciences, Chemistry and Molecular Biology and Biochemistry, University of California, Irvine, CA 92697 Edited by Dinshaw J. Patel, Memorial Sloan-Kettering Cancer Center, New York, NY, and approved July 11, 2011 (received for review May 4, 2011) Multiple lines of evidence support the hypothesis that the early manner, from mixtures of chemically diverse monomer building evolution of life was dominated by RNA, which can both transfer blocks. information from generation to generation through replication Any genetic polymer capable of Darwinian evolution must directed by base-pairing, and carry out biochemical activities by be able to replicate and must be able to encode useful functions. folding into functional structures. To understand how life emerged Of these two properties, replication is probably more tolerant of from prebiotic chemistry we must therefore explain the steps that structural heterogeneity in the sugar-phosphate backbone of a led to the emergence of the RNA world, and in particular, the synth- nucleic acid, because the key to information transfer during esis of RNA. The generation of pools of highly pure ribonucleotides replication is the complementarity of the nucleobases, which on the early Earth seems unlikely, but the presence of alternative act as molecular recognition modules that are largely indepen- nucleotides would support the assembly of nucleic acid polymers dent of the backbone structure (8, 9). -

Front Matter Template
Copyright by Matthew Levy 2003 Design and Evolution of Functional Nucleic Acids by Matthew Levy, B.S., M.S. Dissertation Presented to the Faculty of the Graduate School of The University of Texas at Austin in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy The University of Texas at Austin May, 2003 For Harry, my mother and my father. Dad, now I, too, have advanced graduate degrees. Acknowledgements I would like to thank Andy and all of the members of the Ellington lab, past and present. In particular, I’d like to thank Michael Robertson, who not only first helped show me how to do science in Stanley Miller’s lab, but helped me learn molecular biology in Andy’s lab. I would also like to thank Amos Yan, Jay “Buddy” Hesselberth, Scott Knudsen, Colin “Bunny” Cox, Jeff Tabor and Kristin Thompson for all of their help and fruitful scientific conversations. In addition, I’d like to thank Dr. Kenneth A. Johnson for his assistance with kinetics. Also, a special thanks to Stanley L. Miller for first teaching me how to do research in his lab and sparking my interest in origins research. Finally, I’d like to thank all of my friends in Texas, San Diego and New York, as well as my family, especially my mother Judy, father Walter, brother Alex, stepmother Gene, stepsisters Kate and Kristie (Matt and Emma, too) and stepfather Harvey, for all of their love and support over the years. v Design and Evolution of Functional Nucleic Acids Publication No._____________ Matthew Levy, Ph. -

Stereoselective Prebiotic Nucleotide Synthesis for Threose Nucleic Acid
XVIIIth Intl Conf on Origin of Life 2017 (LPI Contrib. No. 1967) 4184.pdf July 16-21, 2017 at UC San Diego, CA, USA Stereoselective Prebiotic Nucleotide Synthesis for Threose Nucleic Acid Hyo-Joong Kim Firebird Biomolecular Sciences LLC, 13709 Progress Blvd., Alachua, FL 32615 * [email protected] The glycosidic bond formation from sugar and nucleic bases is one of the fundamental problems in prebiotic chemistry for the RNA world hypothesis. However, nucleoside synthesis from sugar and nucleobases has not been successful except the marginal success by Orgel and his coworkers.1 Recently two studies (Sutherland2 and Carell3 groups) showed pyrimidine and purine nucleotide and nucleoside can be synthesized in plausible prebiotic condition. In those syntheses, the nucle- obases were formed after their precursors were attached to sugar then converted to ribo-nucleotide or nucleoside. Threose nucleic acid (TNA) is considered to be one of the plausible prebiotic genetic polymers.4 In search of the prebiotic pathway of TNA building block (threose nucleoside or nucleotide), the reaction of threose and adenine was investigated. The reaction of threose and adenine gave con- densation products in very high yield as an anomeric mixture (more than 70%) but the reaction occurs at the amino-group of the adenine. However the reaction of threose-1,2-cyclic phosphate and adenine provided threose-adenine nucleoside-2’-phosphate stereoselectively (Fig 1). This re- action did not proceed without divalent metal ions (Mg or Ca) which were almost certainly avail- able prebiotically. The prebiotic synthetic pathway of threose-1,2-cyclic phosphate is well known in the literature.5 The -hydroxy aldehyde can be phosphorylated by amidotriphosphate in the presence of magnesium chloride.