Vaccine Safety: the Importance of Going Beyond Passive Surveillance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(MMRV) Vaccine
Addendum to MMR Vaccine (Measles, Mumps, Rubella, and Varicella): What You Need to Know Vaccine Information Statement 1. I agree that the person named below will get the vaccine checked below. 2. I received or was offered a copy of the Vaccine Information Statement (VIS) for the vaccine listed above. 3. I know the risks of the disease this vaccine prevents. 4. I know the benefits and risks of the vaccine. 5. I have had a chance to ask questions about the disease the vaccine prevents, the vaccine, and how the vaccine is given. 6. I know that the person named below will have the vaccine put in his/her body to prevent the disease this vaccine prevents. 7. I am an adult who can legally consent for the person named below to get the vaccine. I freely and voluntarily give my signed permission for this vaccine. Vaccine to be given: Measles, Mumps, Rubella, and Varicella (MMRV) Vaccine Information about person to receive vaccine (Please print) Name: Last First Middle Initial Birthdate Sex (mm/dd/yy) (circle one) M F Address: Street City County State Zip TX Signature of person to receive vaccine or person authorized to make the request (parent or guardian): x Date: x Date: Witness PRIVACY NOTIFICATION - With few exceptions, you have the right to request and be informed about information that the State of Texas collects about you. You are entitled to receive and review the information upon request. You also have the right to ask the state agency to correct any information that is determined to be incorrect. -

Mmrv Vaccine
VACCINE INFORMATION STATEMENT (Measles, Mumps, Many Vaccine Information Statements are available in Spanish and other languages. MMRV Vaccine Rubella and See www.immunize.org/vis Varicella) Hojas de información sobre vacunas están disponibles en español y en muchos otros What You Need to Know idiomas. Visite www.immunize.org/vis These are recommended ages. But children can get the Measles, Mumps, Rubella and second dose up through 12 years as long as it is at least 1 Varicella 3 months after the first dose. Measles, Mumps, Rubella, and Varicella (chickenpox) can be serious diseases: Children may also get these vaccines as 2 separate shots: MMR (measles, mumps and rubella) and Measles varicella vaccines. • Causes rash, cough, runny nose, eye irritation, fever. • Can lead to ear infection, pneumonia, seizures, brain 1 Shot (MMRV) or 2 Shots (MMR & Varicella)? damage, and death. • Both options give the same protection. Mumps • One less shot with MMRV. • Causes fever, headache, swollen glands. • Children who got the first dose as MMRV have • Can lead to deafness, meningitis (infection of the brain had more fevers and fever-related seizures (about and spinal cord covering), infection of the pancreas, 1 in 1,250) than children who got the first dose as painful swelling of the testicles or ovaries, and, rarely, separate shots of MMR and varicella vaccines on death. the same day (about 1 in 2,500). Rubella (German Measles) Your doctor can give you more information, • Causes rash and mild fever; and can cause arthritis, including the Vaccine Information Statements for (mostly in women). MMR and Varicella vaccines. -

Vaccines and Autism: What You Should Know | Vaccine Education
Q A Vaccines and Autism: What you should know Volume& 1 Summer 2008 Some parents of children with autism are concerned that vaccines are the cause. Their concerns center on three areas: the combination measles-mumps-rubella (MMR) vaccine; thimerosal, a mercury-containing preservative previously contained in several vaccines; and the notion that babies receive too many vaccines too soon. Q. What are the symptoms of autism? Q. Does the MMR vaccine cause autism? A. Symptoms of autism, which typically appear during the A. No. In 1998, a British researcher named Andrew Wakefi eld fi rst few years of life, include diffi culties with behavior, social raised the notion that the MMR vaccine might cause autism. skills and communication. Specifi cally, children with autism In the medical journal The Lancet, he reported the stories of may have diffi culty interacting socially with parents, siblings eight children who developed autism and intestinal problems and other people; have diffi culty with transitions and need soon after receiving the MMR vaccine. To determine whether routine; engage in repetitive behaviors such as hand fl apping Wakefi eld’s suspicion was correct, researchers performed or rocking; display a preoccupation with activities or toys; a series of studies comparing hundreds of thousands of and suffer a heightened sensitivity to noise and sounds. children who had received the MMR vaccine with hundreds Autism spectrum disorders vary in the type and severity of of thousands who had never received the vaccine. They found the symptoms they cause, so two children with autism may that the risk of autism was the same in both groups. -

Vaccine Information for PARENTS and CAREGIVERS
NATIONAL INSTITUTE FOR COMMUNICABLE DISEASES Division of the National Health Laboratory Service VACCINE INFOR MATION FOR PARENTS & CAREGIVERS First Edition November 2016 Editors-in-Chief Nkengafac Villyen Motaze (MD, MSc, PhD fellow), Melinda Suchard (MBBCh, FCPath (SA), MMed) Edited by: Cheryl Cohen, (MBBCh, FCPath (SA) Micro, DTM&H, MSc (Epi), Phd) Lee Baker, (Dip Pharm) Lucille Blumberg, (MBBCh, MMed (Micro) ID (SA) FFTM (RCPS, Glasgow) DTM&H DOH DCH) Published by: Ideas Wise and Wonderful (IWW) for National Institute for Communicable Diseases (NICD) First Edition: Copyright © 2016 Contributions by: Clement Adu-Gyamfi (BSc Hons, MSc), Jayendrie Thaver (BSc), Kerrigan McCarthy, (MBBCh, FCPath (SA), DTM&H, MPhil (Theol) Kirsten Redman (BSc Hons), Nishi Prabdial-Sing (PhD), Nonhlanhla Mbenenge (MBBCh, MMED) Philippa Hime (midwife), Vania Duxbury (BSc Hons), Wayne Howard (BSc Hons) Acknowledgments: Amayeza, Vaccine Information Centre (http://www.amayeza-info.co.za/) Centre for Communicable Diseases Fact Sheets (http://www.cdc.gov/vaccines/hcp/vis/) World Health Organization Fact Sheets (http://www.who.int/mediacentre/factsheets/en/) National Department of Health, South Africa (http://www.health.gov.za/) Disclaimer This book is intended as an educational tool only. Information may be subject to change as schedules or formulations are updated. Summarized and simplified information is presented in this booklet. For full prescribing information and contraindications for vaccinations, please consult individual package inserts. There has been -

Combination Vaccines
INFORMATION FOR PARENTS Combination Vaccines Last updated April 2014 The measles, mumps, and rubella vaccine (MMR) and Combination vaccines reduce the diphtheria, tetanus, and pertussis vaccine (DTaP) each protect your child against three diseases. However, these number of shots your child needs two vaccines are not considered true combination vaccines while protecting against several because in the United States, you cannot get separate serious diseases. vaccines for all of the diseases that MMR and DTaP protect against. Fewer Shots—Same Protection Some examples of common combination vaccines for children are: Combination vaccines take two or more vaccines that could be given individually and put them into one • Comvax, which combines Hib and Hep B shot. Children get the same protection as they do from • Twinrix, which combines Hep A and Hep B individual vaccines given separately—but with fewer shots. • Pediarix, which combines DTaP, Hep B, and IPV (polio) So, at a doctor’s visit, your child may only get two or three shots to protect him from five diseases, instead of five • ProQuad, which combines MMR and individual shots. Fewer shots may mean less pain for your varicella (chickenpox) child and less stress for you. • Kinrix, which combines DTaP and IPV (polio) • Pentacel, which combines DTaP, IPV (polio), and Hib To learn about the diseases that vaccines prevent, visit: http://www.cdc.gov/vaccines/vpd-vac/fact-sheet-parents.html Fewer Shots—On Time Protection Combination vaccines help parents, doctors, and nurses keep children up-to-date on vaccines. Combining vaccines into fewer shots may mean that more children will get recommended vaccinations on time. -

Section Only
Figure 1. Recommended Immunization Schedule for Children with HIV Infection Aged 0 through 18 Years; United States, 2019 (Last updated October 25, 2019; last reviewed October 25, 2019) ▲ 1 2 4 6 9 12 13 15 18 19-23 2-3 4-6 7-10 13-14 15 16-18 Vaccine ▼ Age Birth 11-12 yrs month months months months months months months months months months yrs yrs yrs yrs yrs yrs See Hepatitis B1 (Hep B) Hep B Hep B Hep B Hep B Series footnote 1 Rotavirus2 (RV) RV1 (2-dose series); RV5 (3 RV RV RV dose-series) Diphtheria, tetanus & acellular pertussis3 (DTaP: DTaP DTaP DTaP DTaP DTaP DTaP DTaP <7 yrs) Haemophilus influenzae Hib Hib Hib Hib Hib Hib type b4 (Hib) Pneumococcal conjugate5 PCV13 PCV13 PCV13 PCV13 PCV13 PCV13 PCV13 (PCV13) Inactivated poliovirus6 IPV IPV IPV IPV IPV IPV IPV IPV IPV (IPV:<18 yrs) IIV Influenza7 (IIV) IIV (Yearly) (Yearly) Measles, mumps, rubella8 MMR MMRMMR MMR MMR MMR (MMR) Do not administer to severely immunocompromised children Var Var Var Varicella Varicella Varicella9 (Var) Do not administer to severely immunocompromised children Hep A Hepatitis A10 (HepA) Hep A (2 doses) Hep A Series Series Meningococcal11 (MenACWY-D ≥9 mos; MenACWY-CRM (through 23 months), MenACWY-D (9 months through 23 months) MenACWY MedACWY-CRM ≥2 mos) Tetanus, diphtheria, & acellular pertussis12 TdapTdap Tdap Tdap Tdap (Tdap: ≥7 years) Human Papillomavirus13 HPV HPV (3 (9vHPV: males and (3 HPV (3 doses) doses) females) doses) Meningococcal B11 MenB Pneumococcal PPSV23 polysaccharide (PPSV23) Range of recommended ages for vaccination Catch-up immunization Certain high-risk groups Guidelines for the Prevention and Treatment of Opportunistic Infections In HIV-Exposed and HIV-Infected Children JJ-1 Figure 1. -

Vaccine Information Statement
VACCINE INFORMATION STATEMENT Many Vaccine Information Statements are available in Spanish and other languages. MMRV (Measles, Mumps, Rubella, and See www.immunize.org/vis Hojas de información sobre vacunas están Varicella) Vaccine: What You Need to Know disponibles en español y en muchos otros idiomas. Visite www.immunize.org/vis 1 Why get vaccinated? 2 MMRV Vaccine Measles, mumps, rubella, and varicella are viral diseases that can MMRV vaccine may be given to children 12 months through 12 have serious consequences. Before vaccines, these diseases were years of age. Two doses are usually recommended: very common in the United States, especially among children. First dose: 12 through 15 months of age They are still common in many parts of the world. Second dose: 4 through 6 years of age Measles A third dose of MMR might be recommended in certain mumps Measles virus causes symptoms that can include fever, cough, outbreak situations. runny nose, and red, watery eyes, commonly followed by a rash There are no known risks to getting MMRV vaccine at the same that covers the whole body. time as other vaccines. Measles can lead to ear infections, diarrhea, and infection of the lungs (pneumonia). Rarely, measles can cause brain damage or Instead of MMRV, some children 12 months death. through 12 years of age might get 2 separate Mumps shots: MMR (measles, mumps and rubella) and Mumps virus causes fever, headache, muscle aches, tiredness, chickenpox (varicella). MMRV is not licensed for loss of appetite, and swollen and tender salivary glands under the people 13 years of age or older. -

MMRV Vaccine
Combination Measles-Mumps-Rubella and Varicella Vaccine (MMRV) PRIORIX-TETRA® Supplier: GlaxoSmithKline Inc. PROQUAD® Supplier: Merck Canada Inc. INDICATIONS: • School entry dose (4-6 years of age). • Susceptible unimmunized or incompletely immunized persons 4-12 years of age. A In BC these vaccines are not routinely recommended in children under 4 years of age as a first dose due to increased risk of febrile seizures. B DOSES AND SCHEDULE: Routinely as a 2nd dose at 4-6 years of age (inclusive): 1 dose given as 0.5 mL SC (see ADMINISTRATION). Unimmunized persons 4-12 years of age (inclusive): 2 doses given as 0.5 mL SC, 12 weeks apart (see ADMINISTRATION). C Incompletely immunized persons 7-12 years of age (inclusive): 1 dose given as 0.5 mL SC (see ADMINISTRATION). ADMINISTRATION: • Both products need to be reconstituted. Use the diluent provided with the vaccine. • PROQUAD®: Administer the entire volume of reconstituted product, which may be 0.5-0.7 mL. • PRIORIX-TETRA®: Administer the entire volume of reconstituted product, which may be 0.5-0.7 mL. BOOSTER DOSES: No booster doses are recommended at this time. SEROLOGICAL TESTING: Serological testing is not routinely recommended before or after immunization. A As of June 2018, a varicella susceptible person is one without a history of lab confirmed varicella or herpes zoster after 12 months of age and without a history of age appropriate varicella immunization. Individuals with a documented exemption in the immunization registry prior to this date due to previous disease will be considered immune. A self-reported history of varicella or physician diagnosed varicella is adequate only if disease occurred before 2004. -
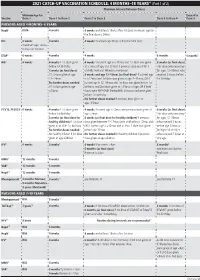
2021 CATCH-UP VACCINATION SCHEDULE: 4 MONTHS–18 YEARS* (Part 1 of 2) Minimum Interval Between Doses
2021 CATCH-UP VACCINATION SCHEDULE: 4 MONTHS–18 YEARS* (Part 1 of 2) Minimum Interval Between Doses Minimum Age for Dose 4 to Vaccine Dose 1 Dose 1 to Dose 2 Dose 2 to Dose 3 Dose 3 to Dose 4 Dose 5 PERSONS AGED 4 MONTHS–6 YEARS HepB1 Birth 4 weeks 8 weeks and at least 16wks after 1st dose; minimum age for the final dose is 24wks RV2 6 weeks 4 weeks 4 weeks maximum age 8mos, 0 days for final dose maximum age 14wks, 6 days for 1st dose DTaP3 6 weeks 4 weeks 4 weeks 6 months 6 months3 Hib4 6 weeks 4 weeks if 1st dose given 4 weeks4 if current age is <12mos and 1st dose was given 8 weeks (as final dose) before 1st birthday at <7mos of age, and at least 1 previous dose was PRP-T This dose only necessary 8 weeks (as final dose) (ActHib, Pentacel, Hiberix) or unknown for ages 12–59mos who if 1st dose given at age 8 weeks and age 12–59mos (as final dose)4 if current age received 3 doses before 12–14mos is <12mos and 1st dose was given at age 7–11mos; OR if 1st birthday No further doses needed current age is 12–59mos and 1st dose was given before 1st if 1st dose given at age birthday, and 2nd dose given at <15mos of age; OR if both ≥15mos doses were PRP-OMP (PedvaxHIB; Comvax) and were given before 1st birthday No further doses needed if previous dose given at age ≥15mos PCV13, PPSV235 6 weeks 4 weeks if 1st dose given 4 weeks if current age <12mos and previous dose given at 8 weeks (as final dose) before 1st birthday age <7mos This dose only necessary 8 weeks (as final dose for 8 weeks (as final dose for healthy children) if previous for ages 12–59mos healthy children) if 1st dose dose given between 7–11mos (wait until at least 12mos old); who received 3 doses given at or after 1st birthday OR if current age is ≥12mos and at least 1 dose was given before age 12mos or No further doses needed before age 12mos. -

MMRV Vaccine Should Not Get Another Dose
VACCINE INFORMATION STATEMENT (Measles, Mumps Many Vaccine Information Statements are available in Spanish and other languages. MMR Vaccine and Rubella) See www.immunize.org/vis Hojas de información sobre vacunas están disponibles en español y en muchos otros What You Need to Know idiomas. Visite www.immunize.org/vis 1 Why get vaccinated? Who should get MMR vaccine 2 and when? Measles, mumps, and rubella are serious diseases. Before vaccines they were very common, especially among Children should get 2 doses of MMR vaccine: children. • First Dose: 12–15 months of age Measles • Second Dose: 4–6 years of age (may be given earlier, if t Measles virus causes rash, cough, runny nose, eye at least 28 days afer the 1st dose) irritation, and fever. Some infants younger than 12 months should get a dose t It can lead to ear infection, pneumonia, seizures of MMR if they are traveling out of the country. (Tis (jerking and staring), brain damage, and death. dose will not count toward their routine series.) Mumps Some adults should also get MMR vaccine: Generally, t Mumps virus causes fever, headache, muscle pain, loss anyone 18 years of age or older who was born afer 1956 of appetite, and swollen glands. should get at least one dose of MMR vaccine, unless they t It can lead to deafness, meningitis (infection of the can show that they have either been vaccinated or had all brain and spinal cord covering), painful swelling of the three diseases. testicles or ovaries, and rarely sterility. MMR vaccine may be given at the same time as other Rubella (German Measles) vaccines. -

Managing Adverse Events Following Immunization: Resource for Public Health
Managing Adverse Events Following Immunization: Resource for Public Health By Special Immunization Clinic Network Investigators of the Canadian Immunization Research Network Version date: 09/01/2019 AEFI Management Resource for Public Health Contents INTRODUCTION .............................................................................................................. 3 LIST OF ABBREVIATIONS AND ACRONYMS ........................................................... 4 ALLERGIC-LIKE EVENTS (WITH OR WITHOUT ANAPHYLAXIS) ........................ 6 APNEA IN PRETERM INFANTS (APTI) ........................................................................ 9 ARTHRALGIA/ARTHRITIS........................................................................................... 10 FEVER .............................................................................................................................. 11 GUILLAIN-BARRÉ SYNDROME (GBS) ...................................................................... 12 HENOCH-SCHONLEIN PURPURA (HSP) ................................................................... 14 HYPOTONIC-HYPORESPONSIVE EPISODE (HHE) .................................................. 15 LOCAL REACTIONS AT THE INJECTION SITE (LRs) ............................................. 16 OCULO-RESPIRATORY SYNDROME (ORS) ............................................................. 19 PERSISTENT CRYING ................................................................................................... 20 SEIZURES ....................................................................................................................... -
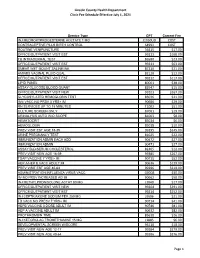
View Revised Fee Schedule
Lincoln County Health Department Clinic Fee Schedule Effective July 1, 2021 Service Type CPT Current Fee INJ MEDROXYPROGESTERNE ACETATE 1 MG J1050UD COST CONTRACEPTIVE PILLS BIRTH CONTROL S4993 COST ROUTINE VENIPUNCTURE 36415 $17.00 OFFICE/OUTPATIENT VISIT EST 99213 $168.00 TB INTRADERMAL TEST 86580 $23.00 OFFICE/OUTPATIENT VISIT EST 99211 $63.00 SMEAR WET MOUNT SALINE/INK 87210 $21.00 AMINES VAGINAL FLUID QUAL 82120 $12.00 OFFICE/OUTPATIENT VISIT EST 99212 $117.00 LIPID PANEL 80061 $38.00 ASSAY GLUCOSE BLOOD QUANT 82947 $15.00 OFFICE/OUTPATIENT VISIT NEW 99203 $267.00 GLYCOSYLATED HEMOGLOBIN TEST 83036 $21.00 IIV4 VACC NO PRSV 3 YRS+ IM 90686 $29.00 RN SERVICES UP TO 15 MINUTES T1002 $51.00 CULTURE SCREEN ONLY 87081 $19.00 URINALYSIS AUTO W/O SCOPE 81003 $8.00 HEMATOCRIT 85014 $6.00 HEMOGLOBIN 85018 $10.00 PREV VISIT EST AGE 18-39 99395 $245.00 URINE PREGNANCY TEST 81025 $22.00 IMMUNIZATION ADMIN EACH ADD 90472 $27.00 IMMUNIZATION ADMIN 90471 $27.00 ASSAY BLD/SERUM CHOLESTEROL 82465 $12.00 PREV VISIT NEW AGE 18-39 99385 $267.00 TDAP VACCINE 7 YRS/> IM 90715 $52.00 HEP A/HEP B VACC ADULT IM 90636 $129.00 PREV VISIT EST AGE 40-64 99396 $319.00 ADMINISTRATION INFLUENZA VIRUS VACC G0008 $30.00 IIV NO PRSV INCREASED AG IM 90662 $58.00 INJ METHYLPRDNISOLONE ACTAT 80 MG J1040 $17.00 OFFICE/OUTPATIENT VISIT NEW 99204 $391.00 OFFICE/OUTPATIENT VISIT EST 99214 $252.00 INJ CEFTRIAXONE SODIUM PER 250 MG J0696 $21.00 TD VACC NO PRESV 7 YRS+ IM 90714 $41.00 HEPB VACCINE 3 DOSE ADULT IM 90746 $81.00 HEP A VACCINE ADULT IM 90632 $82.00 PROTHROMBIN TIME