CMS National Coverage Determination Update
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bris Or Brit Milah (Ritual Circumcision) According to Jewish Law, a Healthy Baby Boy Is Circumcised on the Eighth Day After His Birth
Bris or Brit milah (ritual circumcision) According to Jewish law, a healthy baby boy is circumcised on the eighth day after his birth. The brit milah, the ritual ceremony of removing the foreskin which covers the glans of the penis, is a simple surgical procedure that can take place in the home or synagogue and marks the identification of a baby boy as a Jew. The ceremony is traditionally conducted by a mohel, a highly trained and skilled individual, although a rabbi in conjunction with a physician may perform the brit milah. The brit milah is a joyous occasion for the parents, relatives and friends who celebrate in this momentous event. At the brit milah, it is customary to appoint a kvater (a man) and a kvaterin (a woman), the equivalent of Jewish godparents, whose ritual role is to bring the child into the room for the circumcision. Another honor bestowed on a family member is the sandak, who is most often the baby’s paternal grandfather or great-grandfather. This individual traditionally holds the baby during the circumcision ceremony. The service involves a kiddush (prayer over wine), the circumcision, blessings, a dvar torah (a small teaching of the Torah) and the presentation of the Jewish name selected for the baby. During the brit milah, a chair is set aside for Elijah the prophet. Following the ceremony, a seudat mitzvah (celebratory meal) is available for the guests. Please take note: Formal invitations for a bris are not sent out. Typically, guests are notified by phone or email. The baby’s name is not given before the bris. -

Guidelines on Paediatric Urology S
Guidelines on Paediatric Urology S. Tekgül (Chair), H.S. Dogan, E. Erdem (Guidelines Associate), P. Hoebeke, R. Ko˘cvara, J.M. Nijman (Vice-chair), C. Radmayr, M.S. Silay (Guidelines Associate), R. Stein, S. Undre (Guidelines Associate) European Society for Paediatric Urology © European Association of Urology 2015 TABLE OF CONTENTS PAGE 1. INTRODUCTION 7 1.1 Aim 7 1.2 Publication history 7 2. METHODS 8 3. THE GUIDELINE 8 3A PHIMOSIS 8 3A.1 Epidemiology, aetiology and pathophysiology 8 3A.2 Classification systems 8 3A.3 Diagnostic evaluation 8 3A.4 Disease management 8 3A.5 Follow-up 9 3A.6 Conclusions and recommendations on phimosis 9 3B CRYPTORCHIDISM 9 3B.1 Epidemiology, aetiology and pathophysiology 9 3B.2 Classification systems 9 3B.3 Diagnostic evaluation 10 3B.4 Disease management 10 3B.4.1 Medical therapy 10 3B.4.2 Surgery 10 3B.5 Follow-up 11 3B.6 Recommendations for cryptorchidism 11 3C HYDROCELE 12 3C.1 Epidemiology, aetiology and pathophysiology 12 3C.2 Diagnostic evaluation 12 3C.3 Disease management 12 3C.4 Recommendations for the management of hydrocele 12 3D ACUTE SCROTUM IN CHILDREN 13 3D.1 Epidemiology, aetiology and pathophysiology 13 3D.2 Diagnostic evaluation 13 3D.3 Disease management 14 3D.3.1 Epididymitis 14 3D.3.2 Testicular torsion 14 3D.3.3 Surgical treatment 14 3D.4 Follow-up 14 3D.4.1 Fertility 14 3D.4.2 Subfertility 14 3D.4.3 Androgen levels 15 3D.4.4 Testicular cancer 15 3D.5 Recommendations for the treatment of acute scrotum in children 15 3E HYPOSPADIAS 15 3E.1 Epidemiology, aetiology and pathophysiology -

IS CIRCUMCISION a FRAUD? FRAUD? a IS CIRCUMCISION Peter W
cjp_30-1_42664 Sheet No. 27 Side A 11/12/2020 09:05:36 \\jciprod01\productn\C\CJP\30-1\CJP102.txt unknown Seq: 1 11-NOV-20 14:50 IS CIRCUMCISION A FRAUD? Peter W. Adler, Robert Van Howe, Travis Wisdom & Felix Daase* This Article suggests that non-therapeutic male circumcision or male genital cutting (MGC), the irreversible removal of the foreskin from the penises of healthy boys, is not only unlawful in the United States but also fraudulent. As a German court held in 2012 before its ruling was effectively overturned by a special statute under political pressure, cir- cumcision for religious or non-medical reasons is harmful, violates the child’s rights to bodily integrity and self-determination (which super- sedes competing parental rights), and constitutes criminal assault. MGC also violates the child’s rights under U.S. law, and it constitutes a bat- tery, a tort and a crime, and statutory child abuse. Building upon a 2016 case in the United Kingdom, we make the novel suggestion that when performed by a physician, MGC is a breach of trust or fiduciary duty, and hence constructive fraud, where courts impute fraud even if intent to defraud is absent. We reprise and build upon the argument that it is unlawful and Medicaid fraud for physicians and hospitals to bill Medi- caid for unnecessary genital surgery. Finally, we suggest that MGC con- stitutes intentional fraud by the American Academy of Pediatrics (AAP) and most physicians who perform circumcisions in the United States. They have long portrayed MGC as medicine when it is violence, and as a parental right when males have the right to keep their penile foreskin, and physicians are not allowed to take orders from parents to perform cjp_30-1_42664 Sheet No. -

The Human Foreskin the Foreskin Is Not an Optional Extra for a Man’S Body, Or an Accident
The Human Foreskin The foreskin is not an optional extra for a man’s body, or an accident. It is an integral, functioning, important component of a man’s penis. An eye does not function properly without an eyelid – and nor does a penis without its foreskin. Among other things, the foreskin provides: Protection The foreskin fully covers the glans (head) of the flaccid penis, thereby protecting it from damage and harsh rubbing against abrasive agents (underwear, etc.) and maintaining its sensitivity Sexual Sensitivity The foreskin provides direct sexual pleasure in its own right, as it contains the highest concentration of nerve endings on the penis Lubrication The foreskin, with its unique mucous membrane, permanently lubricates the glans, thus improving sensitivity and aiding smoother intercourse Skin-Gliding During Erection The foreskin facilitates the gliding movement of the skin of the penis up and down the penile shaft and over the glans during erection and sexual activity Varied Sexual Sensation The foreskin facilitates direct stimulation of the glans during sexual activity by its interactive contact with the sensitive glans Immunological Defense The foreskin helps clean and protect the glans via the secretion of anti-bacterial agents What circumcision takes away The foreskin is at the heart of male sexuality. Circumcision almost always results in a diminution of sexual sensitivity; largely because removing the foreskin cuts away the most nerve-rich part of the penis (up to 80% of the penis’s nerve endings reside in the foreskin) [1]. The following anatomy is amputated with circumcision: The Taylor “ridged band” (sometimes called the “frenar band”), the primary erogenous zone of the male body. -

Thromboprophylaxis in Urological Surgery
EAU Guidelines on Thromboprophylaxis in Urological Surgery K.A.O. Tikkinen (Chair), R. Cartwright, M.K. Gould, R. Naspro, G. Novara, P.M. Sandset, P. D . Violette, G.H. Guyatt © European Association of Urology 2018 TABLE OF CONTENTS PAGE 1. INTRODUCTION 3 1.1 Aims and objectives 3 1.2 Panel composition 3 1.3 Available publications 3 1.4 Publication history 3 2. METHODS 3 2.1 Guideline methodology 3 3. GUIDELINE 4 3.1 Thromboprophylaxis post-surgery 4 3.1.1 Introduction 4 3.1.2 Outcomes and definitions 4 3.1.3 Timing and duration of thromboprophylaxis 4 3.1.4 Basic principles for recommending (or not recommending) post-surgery thromboprophylaxis 5 3.1.4.1 Effect of prophylaxis on key outcomes 5 3.1.4.2 Baseline risk of key outcomes 5 3.1.4.3 Patient-related risk (and protective) factors 5 3.1.4.4 From evidence to recommendations 6 3.1.5 General statements for all procedure-specific recommendations 7 3.1.6 Recommendations 7 3.2 Peri-operative management of antithrombotic agents in urology 14 3.2.1 Introduction 14 3.2.2 Evidence summary 14 3.2.3 Recommendations 14 4. RESEARCH RECOMMENDATIONS 16 5. REFERENCES 16 6. CONFLICT OF INTEREST 18 7. ACKNOWLEDGEMENTS 18 8. CITATION INFORMATION 18 2 THROMBOPROPHYLAXIS - MARCH 2017 1. INTRODUCTION 1.1 Aims and objectives Due to the hypercoagulable state induced by surgery, serious complications of urological surgery include deep vein thrombosis (DVT) and pulmonary embolism (PE) - together referred to as venous thromboembolism (VTE) - and major bleeding [1-4]. -

The Circumcision Decision
The Circumcision Decision Circumcision is a surgical procedure in which the skin covering the end of the penis is removed. Scientific studies show a number of medical benefits to circumcision. Parents may also want their son circumcised for religious, social or cultural reasons. Because circumcision is not essential to a child’s health, parents should choose what is best for their child by looking at the benefits, the risks and discussing it with their pediatrician. The following are answers to common questions about circumcision. What Is Circumcision? What Should I Expect For My Son After Circumcision? At birth, boys have skin that covers the end of the penis, called the foreskin. After the circumcision, the tip of the penis Circumcision surgically removes the may seem raw or yellowish. The diaper foreskin, exposing the tip of the penis. should be applied loosely and changed Circumcision is usually performed by a frequently for the first few days. This doctor in the first few days of life. An will help prevent possible infection and infant must be stable and healthy to safely keep the penis from becoming irritated. be circumcised. Petroleum jelly may be used to keep the healing penis from sticking to the Because circumcision may be more risky diaper. Surgical gauze is often applied if done later in life, parents should decide after completion of the circumcision to before or soon after their son is born if prevent bleeding. The gauze will fall off they want it done. on its own in a few days. The penis should be fully healed in about 7-10 days after Is Circumcision Painful? circumcision. -
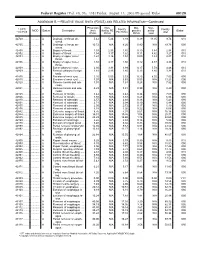
RELATIVE VALUE UNITS (RVUS) and RELATED INFORMATION—Continued
Federal Register / Vol. 68, No. 158 / Friday, August 15, 2003 / Proposed Rules 49129 ADDENDUM B.—RELATIVE VALUE UNITS (RVUS) AND RELATED INFORMATION—Continued Physician Non- Mal- Non- 1 CPT/ Facility Facility 2 MOD Status Description work facility PE practice acility Global HCPCS RVUs RVUs PE RVUs RVUs total total 42720 ....... ........... A Drainage of throat ab- 5.42 5.24 3.93 0.39 11.05 9.74 010 scess. 42725 ....... ........... A Drainage of throat ab- 10.72 N/A 8.26 0.80 N/A 19.78 090 scess. 42800 ....... ........... A Biopsy of throat ................ 1.39 2.35 1.45 0.10 3.84 2.94 010 42802 ....... ........... A Biopsy of throat ................ 1.54 3.17 1.62 0.11 4.82 3.27 010 42804 ....... ........... A Biopsy of upper nose/ 1.24 3.16 1.54 0.09 4.49 2.87 010 throat. 42806 ....... ........... A Biopsy of upper nose/ 1.58 3.17 1.66 0.12 4.87 3.36 010 throat. 42808 ....... ........... A Excise pharynx lesion ...... 2.30 3.31 1.99 0.17 5.78 4.46 010 42809 ....... ........... A Remove pharynx foreign 1.81 2.46 1.40 0.13 4.40 3.34 010 body. 42810 ....... ........... A Excision of neck cyst ........ 3.25 5.05 3.53 0.25 8.55 7.03 090 42815 ....... ........... A Excision of neck cyst ........ 7.07 N/A 5.63 0.53 N/A 13.23 090 42820 ....... ........... A Remove tonsils and ade- 3.91 N/A 3.63 0.28 N/A 7.82 090 noids. -

The Effect of Circumcision on the Mental Health of Children: a Review •
Turkish Journal of Psychiatry 2012 Th e Eff ect of Circumcision on the Mental Health of Children: A Review • Mesut YAVUZ1, Türkay DEMİR2, Burak DOĞANGÜN2 SUMMARY Circumcision is one of the oldest and most frequently performed surgical procedures in the world. It is thought that the beginning of the male circumcision dates back to the earliest times of history. Approximately 13.3 million boys and 2 million girls undergo circumcision each year. In western societies, circumcision is usually performed in infancy while in other parts of the world, it is performed at different developmental stages. Each year in Turkey, especially during the summer months, thousands of children undergo circumcision. The motivations for circumcision include medical-therapeutic, preventive-hygienic and cultural reasons. Numerous publications have suggested that circumcision has serious traumatic ef- fects on children’s mental health. Studies conducted in Turkey draw attention to the positive meanings attributed to the circumcision in the com- munity and emphasize that social effects limit the negative effects of circumcision. Although there are many publications in foreign literature about the mental effects of the circumcision on children’s mental health, there are only a few studies in Turkey about the mental effects of the one of the most frequently performed surgical procedures in our country. The aim of this study is to review this issue. The articles related to circumcision were searched by keywords in Pubmed, Medline, EBSCHOHost, PsycINFO, Turkish Medline, Cukurova Index Database and in Google Scholar and those appropriate for this review were used by authors. Key Words: Circumcision, child, mental health, psychology, trauma INTRODUCTION the Pacific (WHO 2006). -

Male Circumcision J Med Ethics: First Published As 10.1136/Jme.2003.003921 on 1 June 2004
SYMPOSIUM ON CIRCUMCISION 241 device is now off patent, and could be Male circumcision J Med Ethics: first published as 10.1136/jme.2003.003921 on 1 June 2004. Downloaded from ....................................................................................... mass produced at very low cost. Even if the rest of the world continues to reject male circumcision, there are a Male circumcision: a scientific billion Muslims for whom it will remain a fact of life. Islamic nations such as perspective Egypt, Sudan, Iraq, Iran, Pakistan, Bangladesh, and Indonesia have specta- R V Short cularly low rates of HIV infection com- pared with their neighbours, due at least ................................................................................... in part to male circumcision. Now would be an opportune time for the Western The health benefits of male circumcision are wide ranging world to profit from this Islamic experi- ence, while offering to help them n this issue, John Hutson has re- prospects, is it ethical to dismiss a improve their surgical procedures. iterated the conventional Western simple prophylactic surgical procedure If we believe in evidence based Imedical view that ‘‘the surgical argu- that can halve male rates of infection? medicine, then there can be no debate ment for circumcision of all neonatal The case for male circumcision has about male circumcision; it has become males at present is very weak’’ and he been further strengthened by a recent a desirable option for the whole world. criticises many of the circumcisions multinational case controlled study in Paradoxically, this simple procedure is a performed in later childhood, without developed and developing countries in life saver; it can also bring about major anaesthesia, as ‘‘physically cruel and Europe, Asia, and South America, which improvements to both male and female potentially dangerous’’ [see page has shown that circumcised men are reproductive health. -

Public Use Data File Documentation
Public Use Data File Documentation Part III - Medical Coding Manual and Short Index National Health Interview Survey, 1995 From the CENTERSFOR DISEASECONTROL AND PREVENTION/NationalCenter for Health Statistics U.S. DEPARTMENTOF HEALTHAND HUMAN SERVICES Centers for Disease Control and Prevention National Center for Health Statistics CDCCENTERS FOR DlSEASE CONTROL AND PREVENTlON Public Use Data File Documentation Part Ill - Medical Coding Manual and Short Index National Health Interview Survey, 1995 U.S. DEPARTMENT OF HEALTHAND HUMAN SERVICES Centers for Disease Control and Prevention National Center for Health Statistics Hyattsville, Maryland October 1997 TABLE OF CONTENTS Page SECTION I. INTRODUCTION AND ORIENTATION GUIDES A. Brief Description of the Health Interview Survey ............. .............. 1 B. Importance of the Medical Coding ...................... .............. 1 C. Codes Used (described briefly) ......................... .............. 2 D. Appendix III ...................................... .............. 2 E, The Short Index .................................... .............. 2 F. Abbreviations and References ......................... .............. 3 G. Training Preliminary to Coding ......................... .............. 4 SECTION II. CLASSES OF CHRONIC AND ACUTE CONDITIONS A. General Rules ................................................... 6 B. When to Assign “1” (Chronic) ........................................ 6 C. Selected Conditions Coded ” 1” Regardless of Onset ......................... 7 D. When to Assign -

Older Child, Adolescent, Adult Circumcision & Penile Frenulectomy
Older Child, Adolescent, Adult Circumcision & Penile Frenulectomy Patient Information No-Scalpel No-Needle Vasectomy Circumcision For All Ages Penile Frenulectomy Erectile Dysfunction Peyronie’s Disease Premature Ejaculation Testosterone Assessment Penile Enlargement Minds that innovate. Hands that heal. Hearts that care. No matter what your needs are, we’re in this together. You are considering to have, or about to have, a circumcision or frenulectomy procedure. This booklet will give you the general information you need. Please read it with care, and we hope that you find it to be a useful reference before and after your procedure. * IMPORTANT : If your doctor gives you different advice from what has been provided in this booklet, please follow the specific directions you receive. Contact #304 - 625 Fifth Avenue New Westminster, BC #1101 - 805 West Broadway Vancouver, BC Telephone : 604-717-6200 Fax : 604-526-8952 For Emergencies Only : Dr. Chang : 778-866-1851 Dr. Pollock : 604-644-5775 For General Inquiries : [email protected] Dr. Chang : [email protected] Dr. Pollock : [email protected] www.pollockclinics.com Contents About Us 8 Our Doctors 9 Dr. Neil Pollock, MD 9 Dr. Jack Chang, MD 9 About Circumcision Getting Started 10 What Is Circumcision? 10 What Is The Ideal Age For Circumcision? 10 What Are The Potential Benefits Of Circumcision? 10 What Are The Risks Of Circumcision? 11 Do I Need a Physician Referral In Order To Book An Appointment? 11 Circumcision For Older Children, Adolescents And Adults 12 The Older -

A History of Circumcision Tisa Tollenaar Iowa State University
Winter 2017 Article 4 March 2017 The ncU ut Story: A History of Circumcision Tisa Tollenaar Iowa State University Alixandria Collins Iowa State University Follow this and additional works at: http://lib.dr.iastate.edu/ethos Part of the History of Science, Technology, and Medicine Commons Recommended Citation Tollenaar, Tisa and Collins, Alixandria (2017) "The ncU ut Story: A History of Circumcision," Ethos: Vol. 2017 , Article 4. Available at: http://lib.dr.iastate.edu/ethos/vol2017/iss2/4 This Article is brought to you for free and open access by the Iowa State University Magazines and News at Iowa State University Digital Repository. It has been accepted for inclusion in Ethos by an authorized editor of Iowa State University Digital Repository. For more information, please contact [email protected]. The Uncut Story: A History of Circumcision THE RELIGIOUS, SOCIAL AND SEXUAL BACKGROUND OF THE PRACTICE BY TISA TOLLENAAR DESIGN ALIXANDRIA COLLINS “Are you circumcised?” is a hell of an icebreaker — After that, Clark was admitted as an However, he says it does not explain why the corona [the flesh that joins the shaft approaching men that way is bound to get you some outpatient to the hospital and underwent men have marked themselves in an area and head of the penis].” the surgery. He says it took thirty minutes not publicly shown. He does say that The medical community is torn on their funny looks, to say the least. It’s something that — “if that.” However, recovery wasn’t such marking a “sensitive area” would show views on the procedure, but the American nobody talks about in the open and there’s probably a breeze.