Rho and Ras Gtpases in Axon Growth, Guidance, and Branching
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supp Table 6.Pdf
Supplementary Table 6. Processes associated to the 2037 SCL candidate target genes ID Symbol Entrez Gene Name Process NM_178114 AMIGO2 adhesion molecule with Ig-like domain 2 adhesion NM_033474 ARVCF armadillo repeat gene deletes in velocardiofacial syndrome adhesion NM_027060 BTBD9 BTB (POZ) domain containing 9 adhesion NM_001039149 CD226 CD226 molecule adhesion NM_010581 CD47 CD47 molecule adhesion NM_023370 CDH23 cadherin-like 23 adhesion NM_207298 CERCAM cerebral endothelial cell adhesion molecule adhesion NM_021719 CLDN15 claudin 15 adhesion NM_009902 CLDN3 claudin 3 adhesion NM_008779 CNTN3 contactin 3 (plasmacytoma associated) adhesion NM_015734 COL5A1 collagen, type V, alpha 1 adhesion NM_007803 CTTN cortactin adhesion NM_009142 CX3CL1 chemokine (C-X3-C motif) ligand 1 adhesion NM_031174 DSCAM Down syndrome cell adhesion molecule adhesion NM_145158 EMILIN2 elastin microfibril interfacer 2 adhesion NM_001081286 FAT1 FAT tumor suppressor homolog 1 (Drosophila) adhesion NM_001080814 FAT3 FAT tumor suppressor homolog 3 (Drosophila) adhesion NM_153795 FERMT3 fermitin family homolog 3 (Drosophila) adhesion NM_010494 ICAM2 intercellular adhesion molecule 2 adhesion NM_023892 ICAM4 (includes EG:3386) intercellular adhesion molecule 4 (Landsteiner-Wiener blood group)adhesion NM_001001979 MEGF10 multiple EGF-like-domains 10 adhesion NM_172522 MEGF11 multiple EGF-like-domains 11 adhesion NM_010739 MUC13 mucin 13, cell surface associated adhesion NM_013610 NINJ1 ninjurin 1 adhesion NM_016718 NINJ2 ninjurin 2 adhesion NM_172932 NLGN3 neuroligin -

Supplementary Table 2
Supplementary Table 2. Differentially Expressed Genes following Sham treatment relative to Untreated Controls Fold Change Accession Name Symbol 3 h 12 h NM_013121 CD28 antigen Cd28 12.82 BG665360 FMS-like tyrosine kinase 1 Flt1 9.63 NM_012701 Adrenergic receptor, beta 1 Adrb1 8.24 0.46 U20796 Nuclear receptor subfamily 1, group D, member 2 Nr1d2 7.22 NM_017116 Calpain 2 Capn2 6.41 BE097282 Guanine nucleotide binding protein, alpha 12 Gna12 6.21 NM_053328 Basic helix-loop-helix domain containing, class B2 Bhlhb2 5.79 NM_053831 Guanylate cyclase 2f Gucy2f 5.71 AW251703 Tumor necrosis factor receptor superfamily, member 12a Tnfrsf12a 5.57 NM_021691 Twist homolog 2 (Drosophila) Twist2 5.42 NM_133550 Fc receptor, IgE, low affinity II, alpha polypeptide Fcer2a 4.93 NM_031120 Signal sequence receptor, gamma Ssr3 4.84 NM_053544 Secreted frizzled-related protein 4 Sfrp4 4.73 NM_053910 Pleckstrin homology, Sec7 and coiled/coil domains 1 Pscd1 4.69 BE113233 Suppressor of cytokine signaling 2 Socs2 4.68 NM_053949 Potassium voltage-gated channel, subfamily H (eag- Kcnh2 4.60 related), member 2 NM_017305 Glutamate cysteine ligase, modifier subunit Gclm 4.59 NM_017309 Protein phospatase 3, regulatory subunit B, alpha Ppp3r1 4.54 isoform,type 1 NM_012765 5-hydroxytryptamine (serotonin) receptor 2C Htr2c 4.46 NM_017218 V-erb-b2 erythroblastic leukemia viral oncogene homolog Erbb3 4.42 3 (avian) AW918369 Zinc finger protein 191 Zfp191 4.38 NM_031034 Guanine nucleotide binding protein, alpha 12 Gna12 4.38 NM_017020 Interleukin 6 receptor Il6r 4.37 AJ002942 -

Supplementary Table 1
Supplementary Table 1. 492 genes are unique to 0 h post-heat timepoint. The name, p-value, fold change, location and family of each gene are indicated. Genes were filtered for an absolute value log2 ration 1.5 and a significance value of p ≤ 0.05. Symbol p-value Log Gene Name Location Family Ratio ABCA13 1.87E-02 3.292 ATP-binding cassette, sub-family unknown transporter A (ABC1), member 13 ABCB1 1.93E-02 −1.819 ATP-binding cassette, sub-family Plasma transporter B (MDR/TAP), member 1 Membrane ABCC3 2.83E-02 2.016 ATP-binding cassette, sub-family Plasma transporter C (CFTR/MRP), member 3 Membrane ABHD6 7.79E-03 −2.717 abhydrolase domain containing 6 Cytoplasm enzyme ACAT1 4.10E-02 3.009 acetyl-CoA acetyltransferase 1 Cytoplasm enzyme ACBD4 2.66E-03 1.722 acyl-CoA binding domain unknown other containing 4 ACSL5 1.86E-02 −2.876 acyl-CoA synthetase long-chain Cytoplasm enzyme family member 5 ADAM23 3.33E-02 −3.008 ADAM metallopeptidase domain Plasma peptidase 23 Membrane ADAM29 5.58E-03 3.463 ADAM metallopeptidase domain Plasma peptidase 29 Membrane ADAMTS17 2.67E-04 3.051 ADAM metallopeptidase with Extracellular other thrombospondin type 1 motif, 17 Space ADCYAP1R1 1.20E-02 1.848 adenylate cyclase activating Plasma G-protein polypeptide 1 (pituitary) receptor Membrane coupled type I receptor ADH6 (includes 4.02E-02 −1.845 alcohol dehydrogenase 6 (class Cytoplasm enzyme EG:130) V) AHSA2 1.54E-04 −1.6 AHA1, activator of heat shock unknown other 90kDa protein ATPase homolog 2 (yeast) AK5 3.32E-02 1.658 adenylate kinase 5 Cytoplasm kinase AK7 -

Regulating Rac in the Nervous System: Molecular Function and Disease Implication of Rac Gefs and Gaps
Hindawi Publishing Corporation BioMed Research International Volume 2015, Article ID 632450, 17 pages http://dx.doi.org/10.1155/2015/632450 Review Article Regulating Rac in the Nervous System: Molecular Function and Disease Implication of Rac GEFs and GAPs Yanyang Bai, Xiaoliang Xiang, Chunmei Liang, and Lei Shi JNU-HKUST Joint Laboratory for Neuroscience and Innovative Drug Research, Jinan University, Guangzhou, Guangdong 510632, China Correspondence should be addressed to Lei Shi; [email protected] Received 23 January 2015; Accepted 6 March 2015 Academic Editor: Akito Tanoue Copyright © 2015 Yanyang Bai et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Rho family GTPases, including RhoA, Rac1, and Cdc42 as the most studied members, are master regulators of actin cytoskeletal organization. Rho GTPases control various aspects of the nervous system and are associated with a number of neuropsychiatric and neurodegenerative diseases. The activity of Rho GTPases is controlled by two families of regulators, guanine nucleotide exchange factors (GEFs) as the activators and GTPase-activating proteins (GAPs) as the inhibitors. Through coordinated regulation by GEFs and GAPs, Rho GTPases act as converging signaling molecules that convey different upstream signals in the nervous system. So far,morethan70membersofeitherGEFsorGAPsofRhoGTPaseshavebeenidentifiedinmammals,butonlyasmallsubset of them have well-known functions. Thus, characterization of important GEFs and GAPs in the nervous system is crucial for the understanding of spatiotemporal dynamics of Rho GTPase activity in different neuronal functions. In this review, we summarize the current understanding of GEFs and GAPs for Rac1, with emphasis on the molecular function and disease implication of these regulators in the nervous system. -

Investigation of Two Early Events in Amyotrophic Lateral Sclerosis
INVESTIGATION OF TWO EARLY EVENTS IN AMYOTROPHIC LATERAL SCLEROSIS -MRNA OXIDATION AND UP-REGULATION OF A NOVEL PROTECTIVE FACTOR MSUR1 DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By Yueming Chang, Bachelor in Medicine *********** The Ohio State University 2007 Dissertation Committee: Approved by Professor Chien-liang Glenn Lin, Advisor Professor Andrej Rotter ------------------------------------------- Professor Arthur H.M. Burghes Advisor Professor John Oberdick The Ohio State Biochemistry Program ABSTRACT Amyotrophic Lateral Sclerosis (ALS) is a fatal neurodegenerative disorder that is characterized by progressive degeneration of motor neurons in the spinal cord, motor cortex and brainstem, which typically results in mortality within 2-5 years after the onset of disease. The cause of disease is unknown in the majority of cases, and there is no cure for ALS of the moment. Approximately 5% of ALS cases are familial, and 15-25% of the familial cases are linked to mutation in the gene encoding the antioxidant enzyme Cu 2+ /Zn 2+ superoxide dismutase (SOD1). Overexpression of some of ALS-linked mutant SOD1 proteins in transgenic mice results in the development of a neurological disorder that resembles ALS patients. The transgenic mice expressing mutant SOD1 (G93A) is a commonly used ALS animal model. This dissertation demonstrates two early events occurred in the pre-symptomatic stage of SOD1 (G93A) mice, including RNA oxidation and up-regulation of a novel protective factor MSUR1 (mutant SOD1-upregulated RNA 1). Accumulating evidence indicates that messenger RNA (mRNA) oxidation may be associated with neuronal deterioration during the process of neurodegeneration. -

Continuously Active Transcriptional Programs Are Required to Build Expansive Serotonergic Axon Architectures
CONTINUOUSLY ACTIVE TRANSCRIPTIONAL PROGRAMS ARE REQUIRED TO BUILD EXPANSIVE SEROTONERGIC AXON ARCHITECTURES By LAUREN JANINE DONOVAN Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy Dissertation Advisor: Evan S. Deneris Department of Neurosciences CASE WESTERN RESERVE UNIVERSITY January 2020 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the thesis/dissertation of Lauren Janine Donovan candidate for the degree of Doctor of Philosophy*. Committee Chair Jerry Silver, Ph.D. Committee Member Evan Deneris, Ph.D. Committee Member Heather Broihier, Ph.D. Committee Member Ron Conlon, Ph.D. Committee Member Pola Philippidou, Ph.D. Date of Defense August 29th, 2019 *We also certify that written approval has been obtained for any proprietary material contained therein. ii TABLE OF CONTENTS List of Figures……………………………………………………………………….….vii Abstract………………………………………….………………………………..….…1 CHAPTER 1. INTRODUCTION………………………………………………...……..3 GENERAL INTRODUCTION TO SEROTONIN………………………………….….4 Serotonin: Discovery and function………………………….……………...4 Serotonin Biosynthesis…………………………..…………………………..6 Manipulation of the serotonin system in humans……………………….6 Human mutations in 5-HT related genes………………………………….9 SEROTONIN NEURON NEUROGENESIS……………..………………………….11 5-HT neuron specification……………..………………………………...…11 Development of 5-HT neurons……………..………………………………13 NEUROANATOMY……………..……………………………………………………..13 Cytoarchitecture ……………..………………………………………………13 Adult Ascending 5-HT axon projection system ………………………..14 -

Signaling from Axon Guidance Receptors
Downloaded from http://cshperspectives.cshlp.org/ on January 4, 2018 - Published by Cold Spring Harbor Laboratory Press Signaling from Axon Guidance Receptors Greg J. Bashaw1 and Ru¨diger Klein2 1Department of Neuroscience, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania 19104 2Max-Planck-Institute of Neurobiology, Department of Molecular Neurobiology, Am Klopferspitz 18, 82152 Munich-Martinsried, Germany Correspondence: [email protected] Determining how axon guidance receptors transmit signals to allow precise pathfinding decisions is fundamental to our understanding of nervous system development and may suggest new strategies to promote axon regeneration after injury or disease. Signaling mech- anisms that act downstream of four prominent families of axon guidance cues—netrins, semaphorins, ephrins, and slits—have been extensively studied in both invertebrate and ver- tebrate model systems. Although details of these signaling mechanisms are still fragmentary and there appears to be considerable diversity in how different guidance receptors regulate the motility of the axonal growth cone, a number of common themes have emerged. Here, we review recent insights into how specific receptors for each of these guidance cues engage downstream regulators of the growth cone cytoskeleton to control axon guidance. enerating precise patterns of connectivity endocytosis and proteolytic processing, influ- Gdepends on the regulated action of con- ence guidance receptor activation and signal- served families of guidance cues and their neu- ing and then discuss how bidirectional links ronal receptors. Activation of specific signaling between receptors and cytoplasmic signaling pathways can promote attraction, repulsion, molecules control axon guidance responses. result in growth cone collapse, or affect the rate of axon extension through signaling events ENDOCYTOSIS that act locally to modulate cytoskeletal dynam- ics in the growth cone. -

SUPPORTING INFORMATION for Regulation of Gene Expression By
SUPPORTING INFORMATION for Regulation of gene expression by the BLM helicase correlates with the presence of G4 motifs Giang Huong Nguyen1,2, Weiliang Tang3, Ana I. Robles1, Richard P. Beyer4, Lucas T. Gray5, Judith A. Welsh1, Aaron J. Schetter1, Kensuke Kumamoto1,6, Xin Wei Wang1, Ian D. Hickson2,7, Nancy Maizels5, 3,8 1 Raymond J. Monnat, Jr. and Curtis C. Harris 1Laboratory of Human Carcinogenesis, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, U.S.A; 2Department of Medical Oncology, Weatherall Institute of Molecular Medicine, John Radcliffe Hospital, University of Oxford, Oxford, U.K.; 3Department of Pathology, University of Washington, Seattle, WA U.S.A.; 4 Center for Ecogenetics and Environmental Health, University of Washington, Seattle, WA U.S.A.; 5Department of Immunology and Department of Biochemistry, University of Washington, Seattle, WA U.S.A.; 6Department of Organ Regulatory Surgery, Fukushima Medical University, Fukushima, Japan; 7Cellular and Molecular Medicine, Nordea Center for Healthy Aging, University of Copenhagen, Denmark; 8Department of Genome Sciences, University of WA, Seattle, WA U.S.A. SI Index: Supporting Information for this manuscript includes the following 19 items. A more detailed Materials and Methods section is followed by 18 Tables and Figures in order of their appearance in the manuscript text: 1) SI Materials and Methods 2) Figure S1. Study design and experimental workflow. 3) Figure S2. Immunoblot verification of BLM depletion from human fibroblasts. 4) Figure S3. PCA of mRNA and miRNA expression in BLM-depleted human fibroblasts. 5) Figure S4. qPCR confirmation of mRNA array data. 6) Table S1. BS patient and control detail. -
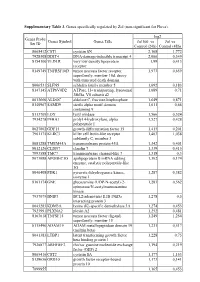
Supplementary Table 3. Genes Specifically Regulated by Zol (Non-Significant for Fluva)
Supplementary Table 3. Genes specifically regulated by Zol (non-significant for Fluva). log2 Genes Probe Genes Symbol Genes Title Zol100 vs Zol vs Set ID Control (24h) Control (48h) 8065412 CST1 cystatin SN 2,168 1,772 7928308 DDIT4 DNA-damage-inducible transcript 4 2,066 0,349 8154100 VLDLR very low density lipoprotein 1,99 0,413 receptor 8149749 TNFRSF10D tumor necrosis factor receptor 1,973 0,659 superfamily, member 10d, decoy with truncated death domain 8006531 SLFN5 schlafen family member 5 1,692 0,183 8147145 ATP6V0D2 ATPase, H+ transporting, lysosomal 1,689 0,71 38kDa, V0 subunit d2 8013660 ALDOC aldolase C, fructose-bisphosphate 1,649 0,871 8140967 SAMD9 sterile alpha motif domain 1,611 0,66 containing 9 8113709 LOX lysyl oxidase 1,566 0,524 7934278 P4HA1 prolyl 4-hydroxylase, alpha 1,527 0,428 polypeptide I 8027002 GDF15 growth differentiation factor 15 1,415 0,201 7961175 KLRC3 killer cell lectin-like receptor 1,403 1,038 subfamily C, member 3 8081288 TMEM45A transmembrane protein 45A 1,342 0,401 8012126 CLDN7 claudin 7 1,339 0,415 7993588 TMC7 transmembrane channel-like 7 1,318 0,3 8073088 APOBEC3G apolipoprotein B mRNA editing 1,302 0,174 enzyme, catalytic polypeptide-like 3G 8046408 PDK1 pyruvate dehydrogenase kinase, 1,287 0,382 isozyme 1 8161174 GNE glucosamine (UDP-N-acetyl)-2- 1,283 0,562 epimerase/N-acetylmannosamine kinase 7937079 BNIP3 BCL2/adenovirus E1B 19kDa 1,278 0,5 interacting protein 3 8043283 KDM3A lysine (K)-specific demethylase 3A 1,274 0,453 7923991 PLXNA2 plexin A2 1,252 0,481 8163618 TNFSF15 tumor necrosis -

Autocrine IFN Signaling Inducing Profibrotic Fibroblast Responses By
Downloaded from http://www.jimmunol.org/ by guest on September 23, 2021 Inducing is online at: average * The Journal of Immunology , 11 of which you can access for free at: 2013; 191:2956-2966; Prepublished online 16 from submission to initial decision 4 weeks from acceptance to publication August 2013; doi: 10.4049/jimmunol.1300376 http://www.jimmunol.org/content/191/6/2956 A Synthetic TLR3 Ligand Mitigates Profibrotic Fibroblast Responses by Autocrine IFN Signaling Feng Fang, Kohtaro Ooka, Xiaoyong Sun, Ruchi Shah, Swati Bhattacharyya, Jun Wei and John Varga J Immunol cites 49 articles Submit online. Every submission reviewed by practicing scientists ? is published twice each month by Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts http://jimmunol.org/subscription Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html http://www.jimmunol.org/content/suppl/2013/08/20/jimmunol.130037 6.DC1 This article http://www.jimmunol.org/content/191/6/2956.full#ref-list-1 Information about subscribing to The JI No Triage! Fast Publication! Rapid Reviews! 30 days* Why • • • Material References Permissions Email Alerts Subscription Supplementary The Journal of Immunology The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2013 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. This information is current as of September 23, 2021. The Journal of Immunology A Synthetic TLR3 Ligand Mitigates Profibrotic Fibroblast Responses by Inducing Autocrine IFN Signaling Feng Fang,* Kohtaro Ooka,* Xiaoyong Sun,† Ruchi Shah,* Swati Bhattacharyya,* Jun Wei,* and John Varga* Activation of TLR3 by exogenous microbial ligands or endogenous injury-associated ligands leads to production of type I IFN. -

Gene Section Review
Atlas of Genetics and Cytogenetics in Oncology and Haematology OPEN ACCESS JOURNAL INIST-CNRS Gene Section Review DOCK1 (Dedicator of cytokinesis 1) Ping Li, Fung Zhao, Annie N. Cheung Departments of Pathology, the University of Hong Kong Shenzhen Hospital, Shenzhen (PL, ANC),, The University of Hong Kong, Hong Kong (FZ, AC), China Published in Atlas Database: March 2015 Online updated version : http://AtlasGeneticsOncology.org/Genes/DOCK1ID40354ch10q26.html Printable original version : http://documents.irevues.inist.fr/bitstream/handle/2042/62524/03-2015-DOCK1ID40354ch10q26.pdf DOI: 10.4267/2042/62524 This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 2.0 France Licence. © 2016 Atlas of Genetics and Cytogenetics in Oncology and Haematology Abstract DNA/RNA Dedicator of cytokinesis (DOCK) is a family of Description proteins with 11 members in mammal which can The DOCK1 gene is 6797 base pairs in length regulate cell motility, phagocytosis, myoblast fusion, encoding a large protein (about 180kDa). tumor suppression, neuronal polarization and adhesion. They are classified into four subfamilies A Transcription to D. Dock1 (Dock180), the founding member of the Variant (1) represents the longer transcript and family, is a large protein which includes an N- encodes the longer isoform (1). terminal SH3 domain and a flanking helical bundle Variant (2) uses an alternate splice site at an internal that are vital to the formation of a functioning exon, compared to variant 1. complex Dock1-ELMO1 (Gumienny et al.,2001; The encoded isoform (2) is shorter, compared to Grimsley et al.,2004; Komander et al., 2008). isoform 1. Genetic and biochemical studies show that DOCK1 acts as a guanine-nucleotide exchange factor (GEF) Protein for the small GTPase Rac1 (Diyokawa et al., 1998; Nolan et al., 1998). -

Dock3 Induces Axonal Outgrowth by Stimulating Membrane Recruitment of the WAVE Complex
Dock3 induces axonal outgrowth by stimulating membrane recruitment of the WAVE complex Kazuhiko Namekataa, Chikako Haradaa,b, Choji Tayac, Xiaoli Guoa, Hideo Kimurad, Luis F. Paradab, and Takayuki Haradaa,b,1 aDepartment of Molecular Neurobiology, Tokyo Metropolitan Institute for Neuroscience, Fuchu, Tokyo 183-8526, Japan; bDepartment of Developmental Biology and Kent Waldrep Foundation Center for Basic Research on Nerve Growth and Regeneration, University of Texas Southwestern Medical Center, Dallas, TX 75390; cDepartment of Laboratory Animal Science, Tokyo Metropolitan Institute of Medical Science, Setagaya, Tokyo 156-8506, Japan; and dDepartment of Molecular Genetics, National Institute of Neuroscience, National Center of Neurology and Psychiatry, Kodaira, Tokyo 187-8502, Japan Edited by Thomas C. Südhof, Stanford University School of Medicine, Palo Alto, CA, and approved March 16, 2010 (received for review December 18, 2009) Atypical Rho-guanine nucleotide exchange factors (Rho-GEFs) that tional interactions (11, 12). Recent studies have demonstrated that contain Dock homology regions (DHR-1 and DHR-2) are expressed in neurotrophins play important roles during gastrulation, in the dif- a variety of tissues; however, their functions and mechanisms of ferentiation of hepatic stellate cells, and in neurulation, through the action remain unclear. We identify key conserved amino acids in the regulation of Rho-GTPases (9, 13, 14). Considering their relatively DHR-2 domain that are critical for the catalytic activity of Dock-GEFs ubiquitous expression patterns, it is conceivable that Dock family (Dock1–4). We further demonstrate that Dock-GEFs directly associ- members, in combination with neurotrophins, could be involved in ate with WASP family verprolin-homologous (WAVE) proteins the development and function of a variety of organs (15–17).