Removal of Plutonium from Uranium by Molten Metal Extraction A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

People and Things
People and things John Cumalat and David Neuffer, first Robert R. Wilson Fellows at Fermilab. The Wilson fellowships are special three year appointments awarded annually at Fermilab to outstanding young physicists in the fields of accelerators and particle physics. On people Accelerator specialist Ernie Courant is the recipient of the 1979 Boris Pregel A ward for Applied Science and Technology. The presentation was made at the annual meeting of the New York Academy of Sciences on 6 December. Giving cancer treatments at TRIUMF The first treatment of cancer patients began at TRIUMF in Nov ember using the negative pion beam from the biomedical channel. In this first series, patients with multiple skin tumour nodules are receiving ten daily treatments. In order to assess the effect of negative pions on human tissue, some of the nodules are being treated with pions and others with X-rays. Only when this is known can treatment of larger, deep-seated tumours com mence. The treatments at TRIUMF have been preceded by comprehen sive pre-clinical investigations in cluding both physical and radiobio logical studies, the latter including cultured cells, mice and pigs. Conferences on the horizon The Sixth International Conference on Experimental Meson Spectro scopy will be held at Brookhaven on 24-25 April. The Conference will cover experimental results in light and heavy quark spectroscopy, rele vant theory and spectrometer sys tems. For further information please contact CU. Chung or S.J. Linden- baum, Brookhaven National Labo ratory, Upton, New York 11973. The Mark II detector, previously used in experiments at SPEAR, seen here being installed in one of the experimental areas of the new PEP electron-positron collider. -
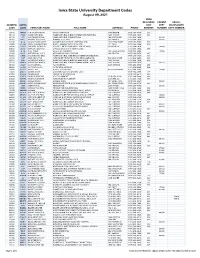
Iowa State University Department Codes
Iowa State University Department Codes August 09, 2021 RMM RESOURCE PARENT CROSS- NUMERIC ALPHA UNIT DEPT DISCIPLINARY CODE CODE DIRECTORY NAME FULL NAME ADDRESS PHONE NUMBER NUMBER DEPT NUMBER 30141 4HFDN 4-H FOUNDATION 4-H FOUNDATION 2150 BDSHR (515) 294-5390 030 01130 A B E AG/BIOSYS ENG AGRICULTURAL & BIOSYSTEMS ENGINEERING 1201 SUKUP (515) 294-1434 001 01132 A E AG ENGINEERING AGRICULTURAL ENGINEERING 100 DAVIDSON (515) 294-1434 01130 01581 A ECL ANIMAL ECOLOGY ANIMAL ECOLOGY 253 BESSEY (515) 294-1458 01580 92290 A I C ACUMEN IND CORP ACUMEN INDUSTRIES CORPORATION 1613 RSRC PARK (515) 296-5366 999 45000 A LAB AMES LABORATORY AMES LABORATORY OF US DOE 151 TASF (515) 294-2680 020 10106 A M D APPAREL MERCH D APPAREL MERCHANDISING AND DESIGN 31 MACKAY (515) 294-7474 10100 80620 A S C APPL SCI COMPUT APPLIED SCIENTIFIC COMPUTING (515) 294-2694 999 10706 A TR ATH TRAIN ATHLETIC TRAINING 235 FORKER BLDG (515) 294-8009 10700 07040 A V C ART/VISUAL CULT ART AND VISUAL CULTURE 146 DESIGN (515) 294-5676 007 70060 A&BE AG & BIOSYS ENG AGRICULTURAL AND BIOSYSTEMS ENGINEERING (515) 294-1434 999 92100 AAT ADV ANAL TCH ADVANCED ANALYTICAL TECHNOLOGIES INC ISU RSRC PARK (515) 296-6600 999 02010 ABE AG/BIOSYS ENG-E AGRICULTURAL & BIOSYSTEMS ENGR - ENGR 1201 SUKUP (515) 294-1434 002 01136 ABE A AG/BIOSYS ENG-A AGRICULTURAL & BIOSYSTEMS ENGR - AGLS 1201 SUKUP (515) 294-1434 01130 08100 ACCT ACCOUNTING ACCOUNTING 2330 GERDIN (515) 294-8106 008 08301 ACSCI ACTUARIAL SCI ACTUARIAL SCIENCE (515) 294-4668 008 10501 AD ED ADULT ED ADULT EDUCATION N131 LAGOMAR -

The United States Nuclear Weapon Program
/.i. - y _-. --_- -. : _ - . i - DOE/ES4005 (Draft) I _ __ _ _ _____-. 67521 - __ __-. -- -- .-- THE UNITED STATES NUCLEAR - %”WEAPQN PROGRA,hik ..I .La;*I* . , ASUMMARYHISTORY \ ;4 h : . ,‘f . March 1983 \ .;_ U.S. Department of Energy Assistant Secretary, Management and Administration Office of The Executive Secretariat History Division -. DOE/ES4005 (Draft) THE UNITED STATES NUCLEAR WEAPON PROG.RAM: ASUMMARYHISTORY .' . c *. By: . Roger M. Anders Archivist With: Jack M. Hall Alice L. Buck Prentice C. Dean March 1983 ‘ .I \ . U.S. Department of Energy Assistant Secretary, Management and Administration Office of The Executive Secretariat History Division Washington, D. C. 20585 ‘Thelkpaemlt of Energy OqanizationAct of 1977 b-mughttcgether for the first tim in one departxrmtrmst of the Federal GovenmTle?t’s - Programs-With these programs cam a score of organizational ‘ . ? entities,eachwithi+ccxmhistoryandtraditions,frmadozendepart- . .‘I w ’ mnts and independentagencies. The EIistoryDivision,- prepareda . seriesof paqhlets on The Institutional Originsof the De-t of v Eachpamphletexplainsthehistory,goals,and achievemzntsof a predecessoragency or a major prqrm of the -to=-TY* This parquet, which replacesF&ger M. Anders'previous booklet on "The Office of MilitaxxApplication," traces the histoe of the UrL+& Statesnuclearweapx prcgramfrmits inceptionduring World War II to the present. Nuclear weqons form the core of America's m&z defenses. Anders'history describes the truly fo&idable effortscf 5e Atanic Energy Cmmission, the F;nergy Rfzsearch and Develqmlt z4dmCstratian,andtheDep&m- to create adiverse a* sophistica~arsenzl ofnucleaz ~accctqli&mentsofL~se agenciesandtheirplants andlabc J zrsatedan "atanic shie2 WMchp- Psrrericatoday. r kger M. Anders is a trained historianworking in the Eistzq Divisbn. -

Department of Energy National Laboratories and Plants: Leadership in Cloud Computing (Brochure), U.S. Department of Energy (DOE)
Department of Energy National Laboratories and Plants Leadership in Cloud Computing Prepared by the National Renewable Energy Laboratory (NREL), a national laboratory of the U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy; NREL is operated by the Alliance for Sustainable Energy, LLC. JJJTABLE OF CONTENTS U.S. DEPARTMENT OF ENERGY NEVADA NATIONAL SECURITY SITE ........................................34 LABORATORIES AND PLANTS ......................................................4 Current State ...............................................................................34 Cloud Vision .................................................................................34 ABOUT THIS REPORT .....................................................................8 Key Initiatives ..............................................................................34 History of Computing ...............................................................9 Evolution of Computing Models ...........................................9 OAK RIDGE NATIONAL LABORATORY ....................................36 What is Cloud Computing? ....................................................9 Current State and Future Work ............................................36 Cloud Security .............................................................................10 RightPath – DOE/NNSA Cloud Strategy ...........................11 PACIFIC NORTHWEST NATIONAL LABORATORY ..............38 Vision ..............................................................................................38 -

The U.S. Department of Energy's Ten-Year-Plans for the Office Of
U.S. DEPARTMENT OF ENERGY The U.S. Department of Energy’s Ten-Year-Plans for the Office of Science National Laboratories FY 2019 FY 2019 Annual Laboratory Plans for the Office of Science National Laboratories i Table of Contents Introduction ................................................................................................................................................................1 Ames Laboratory ........................................................................................................................................................3 Lab-at-a-Glance ......................................................................................................................................................3 Mission and Overview ............................................................................................................................................3 Core Capabilities .....................................................................................................................................................4 Science Strategy for the Future ..............................................................................................................................8 Infrastructure .........................................................................................................................................................8 Argonne National Laboratory ................................................................................................................................. -

Doe-Fy2021-Laboratory-Table 1.Pdf
DOE/CF-0168 Department of Energy FY 2021 Congressional Budget Request Laboratory Tables Preliminary February 2020 Office of Chief Financial Officer DOE/CF-0168 Department of Energy FY 2021 Congressional Budget Request Laboratory Tables Preliminary The numbers depicted in this document represent the gross level of DOE budget authority for the years displayed. The figures include discretionary and supplemental funding. They do not consider revenues/receipts, use of prior year balances, deferrals, rescissions, or other adjustments appropriated as offsets to the DOE appropriations by the Congress. February 2020 Office of Chief Financial Officer Printed with soy ink on recycled paper Table of Contents Laboratory Table by Congressional Control Laboratory Table Summary......................................................................................................................................1 Laboratory Table by Congressional Control4 Ames Laboratory..................................................................................................................................................4 Ames Site Office...................................................................................................................................................5 Argonne National Laboratory...............................................................................................................................6 Argonne Site Office..............................................................................................................................................9 -

The Atomic Energy Commission
The Atomic Energy Commission By Alice Buck July 1983 U.S. Department of Energy Office of Management Office of the Executive Secretariat Office of History and Heritage Resources Introduction Almost a year after World War II ended, Congress established the United States Atomic Energy Commission to foster and control the peacetime development of atomic science and technology. Reflecting America's postwar optimism, Congress declared that atomic energy should be employed not only in the Nation's defense, but also to promote world peace, improve the public welfare, and strengthen free competition in private enterprise. After long months of intensive debate among politicians, military planners and atomic scientists, President Harry S. Truman confirmed the civilian control of atomic energy by signing the Atomic Energy Act on August 1, 1946.(1) The provisions of the new Act bore the imprint of the American plan for international control presented to the United Nations Atomic Energy Commission two months earlier by U.S. Representative Bernard Baruch. Although the Baruch proposal for a multinational corporation to develop the peaceful uses of atomic energy failed to win the necessary Soviet support, the concept of combining development, production, and control in one agency found acceptance in the domestic legislation creating the United States Atomic Energy Commission.(2) Congress gave the new civilian Commission extraordinary power and independence to carry out its awesome responsibilities. Five Commissioners appointed by the President would exercise authority for the operation of the Commission, while a general manager, also appointed by the President, would serve as chief executive officer. To provide the Commission exceptional freedom in hiring scientists and professionals, Commission employees would be exempt from the Civil Service system. -

Ames Laboratory Special Exposure Cohort Petition Evaluation Report
Ames Laboratory Special Exposure Cohort Petition Evaluation Report LaVon B. Rutherford, CHP Special Exposure Cohort Health Physics Team Leader National Institute for Occupational Safety and Health Division of Compensation Analysis and Support JlJuly 2011 1 Petition Overview . March 31, 2011: NIOSH informed an Ames Laboratory claimant that we were unable to reconstruct the radiation dose for the claim . April 7, 2011: NIOSH received an 83.14 SEC petition . April 13, 2011: petition qualified for evaluation . June 9, 2011: NIOSH Evaluation Report issued 2 Petition Overview—cont. NIOSH proposed class to be added to the SEC: All Department of Energy employees, its predecessor agencies, and its contractors and subcontractors who worked in any area of the Ames Laboratory at Iowa State University during the period from January 1 , 1942 through December 31, 1970, for a number of work days aggregating at least 250 work days, occurring either solely under this employment or in combination with work days within the parameters established for one or more classes of employees included in the Special Exposure Cohort. 3 Background . Ames Laboratory is located at Iowa State University in Ames, Iowa. Ames Laboratory is a DOE facility with operations beginning in 1942 and continuing to present. During the World War II years , the primary mission of the Ames Laboratory was the process development and production of uranium and thorium metal. After the war, the mission of the Ames Laboratory shifted to mainly research and development. 4 Background—cont. There currently are three SEC classes associated with the Ames Laboratory: 1. All DOE employees or contractors who workdked at one of the following facilities/locations: Chemistry Annex 1 (also known as “the old women’s gymnasium” and “Little Ankeny ”), Chemistry Annex 2, Chemistry Building (also known as “Gilman Hall”), Research Building, or the Metallurgical Building (also known as “Harley Wilhelm Hall”) from January 1, 1942 through December 31, 1954 (SEC‐0038). -

Research Highlights
Princeton’s Nathaniel Fisch Page 2 Number 169 October 18, 2004 Research HIV dementia mechanism Theory offers new view of the discovered nucleus Highlights . A brain-imaging study at DOE's Scientists have known for more than 30 Brookhaven Lab shows that human years that protons and neutrons are immunodeficiency virus (HIV) damages built of quarks and gluons. But they dopamine-associated brain cells in interpret data from experiments on the patients with HIV dementia—a type of nucleus with "effective theories" which cognitive decline in the later stages of ignore this internal structure for the sake HIV infection. The brain images, of simplicity. Now a new study from obtained with positron emission DOE's Jefferson Lab, together with the tomography (PET) at Brookhaven’s CEA in France, proposes a theory that Center for Translational Neuroimaging, for the first time incorporates the reveal that patients with HIV dementia dynamical changes in the quark-gluon have fewer dopamine transporters— structure of protons and neutrons in the proteins that help to recycle the nucleus. Published Sept. 23 in Physical neurotransmitter dopamine—than HIV- Review Letters, it is a first step towards a negative subjects or HIV-positive patients truly fundamental theory of nuclear without dementia. These findings may structure. lead to new, more effective therapies. [Kandice Carter, 757/269-7263; Future studies will also look at whether [email protected]] treatments to suppress HIV lead to recovery of the dopamine system. [Karen McNulty Walsh, 631/344-8350; [email protected]] Ames research a boon to bioanalysis Studies of individual enzyme molecules New method for medical by Edward S. -

Superconducting Quantum Materials and Systems Center
Fermi National Accelerator LaboratoryFermi National Accelerator Laboratory August 2020 The Superconducting Quantum Materials and Systems Center at Fermilab SQMS brings together world-class experts from 20 institutions to take on one of the biggest challenges in quantum science: extending the lifetimes of quantum states. Advances here will lead to Fermilab and its partners building and operating a revolutionary quantum computer and developing quantum sensors to aid the search for undiscovered particles. The Superconducting Quantum Materials and Systems Center, or SQMS, led by Fermilab, brings together national laboratories, academia and industry to make revolutionary advances in quantum computing and sensing, including the building and deployment of a beyond-the-state-of-the-art quantum computer. The quantum devices developed at SQMS will have game-changing impacts in basic science and in our everyday lives. Longer information lifetimes The driving mission of SQMS is to overcome the biggest barrier to the construction of a quantum computer: the short lifetime of the information that lives in a qubit. Qubits are devices that hold quantum information, analogs of the classical computer bit. Today’s highest-performing qubits maintain information for milliseconds—not long enough for a viable quantum computer. SQMS researchers aim to design and fabricate qubits whose coherence times are thousands of times longer. The specially designed qubits will leverage components called superconducting cavities, which were developed for particle accelerators. Fermilab-developed cavities have achieved coherence times of several seconds. By integrating cutting-edge, industry-designed and -fabricated computer chips into these cavities, SQMS collaborators expect to produce qubits with the longest coherence times ever demonstrated. -

Ames Laboratory U.S
Ames Laboratory U.S. DEPARTMENT OF At a Glance ENERGY Ames Laboratory is DOE’s materials laboratory focused applying this knowledge to solve salient technological and on the design, discovery, fundamental understanding, industrial challenges is a significant strength and notable and application of materials to energy technologies. We focus for Ames. Through the specialized capabilities provide the national and global scientific communities and expertise of our world-renowned research teams, with roadmaps to create and discover new materials. Our we serve the scientific community by making seemingly synthesis, exploration, mapping, and tuning of the physical “impossible” to create materials available to other national properties of materials set the research agenda for the laboratories, universities, and industry—enabling and scientific community for decades to come. The transition accelerating science and technology across the nation. from generating fundamental knowledge of materials to FY 2016 Funding by Source Facts Location: Ames, Iowa Type: Single-program Laboratory Year Founded: 1947 Director: Adam Schwartz Contractor: Iowa State University of Science and Technology Responsible Site Office: Ames Site Office Science, Energy, $25.7M $24.3M Physical Assets 10 acres and 13 buildings 340,968 GSF in buildings Replacement plant value: $88.6M Human Capital 303 full-time equivalent employees (FTEs) 82 joint faculty Other DOE, SPP, 43 postdoctoral researchers $2.1M $1.1M 83 undergraduate students 102 graduate students Lab operating costs: $53.2M 268 visiting scientists DOE/NNSA costs: $52.1M SPP (Non-DOE/Non-DHS) costs: $1.1M SPP as % total Lab operating costs: 2.1% Core Capabilities Mission Unique Facilities • Applied Materials Science and Engineering • Sensitive Instrument Facility • Chemical and Molecular Science • Critical Materials Institute— • Condensed Matter Physics and Materials Science DOE Energy Innovation Hub • Materials Preparation Center • Powder Synthesis Facility for Additive Manufacturing Ames Laboratory U.S. -

Ames Laboratory at a Glance
Ames Laboratory At a Glance Ames Laboratory is a world-class institution dedicated to materials, processes, and technologies that advance the nation’s creating materials, inspiring minds to solve problems, and economic competitiveness and enhance national security. Ames addressing global challenges. For more than 70 years, Ames Laboratory’s location on the campus of its contractor, Iowa State Laboratory has been a leader in the discovery, synthesis, University, has instilled a culture of interdisciplinary science and analysis, and application of new materials, novel chemistries, innovation. Invention of lead-free solder, a hybrid catalyst that and transformational analytical tools. The Laboratory conducts more efficiently converts crops to biofuel, and caloric materials fundamental and applied research that helps the world to better for improved air conditioning and refrigeration are just a few understand the nature of the building blocks that make up our examples of Ames Laboratory’s materials that are impacting universe, and translates that knowledge into new and unique our world. FY 18 Funding by Source ($M) Facts Location: Ames, IA Year Founded: 1947 Director: Adam Schwartz Type: Single-program Laboratory FE, $1.48 Contractor: Iowa State University of Science and Technology EERE, $28.85 Site Office: Ames Site Office Other DOE, Website: www.ameslab.gov $0.13 Other SC, $2.84 SPP, $1.65 Physical Assets BES, $19.57 10 acres and 13 buildings ASCR, $0.08 OE, $0.05 340,968 GSF in buildings FES, $0.07 Replacement Plant Value: $96.6M BER,